ADVERTISEMENTS:
In this article we will discuss about Ustilago. After reading this article you will learn about: 1. Habit and Habitat of Ustilago 2. Symptoms of Ustilago 3. Vegetative Structure 4. Reproduction.
Contents:
- Habit and Habitat of Ustilago
- Symptoms of Ustilago
- Vegetative Structure of Ustilago
- Reproduction in Ustilago
1. Habit and Habitat of Ustilago:
Ustilago, the largest genus of the family Ustilaginaceae is represented by more than 400 cosmopolitan species. Butler and Bisby (1958) reported 108 species from India. All species are parasitic and infect the floral parts of wheat, barley, oat, maize, sugarcane, Bajra, rye and wild grasses.
ADVERTISEMENTS:
The name Ustilago has been derived from a Latin word ustus meaning ‘burnt’ because the members of the genus produce black, sooty powdery mass of spores on the host plant parts imparting them a ‘burnt’ appearance. This black dusty mass of spores resembles soot or smut, therefore, commonly it is also known as smut fungus.
The fungus is of much economic importance, because it causes heavy loss to various economically important plants. This genus is very common in U.P., Bihar, Punjab and Madhya Pradesh.
2. Symptoms of Ustilago:
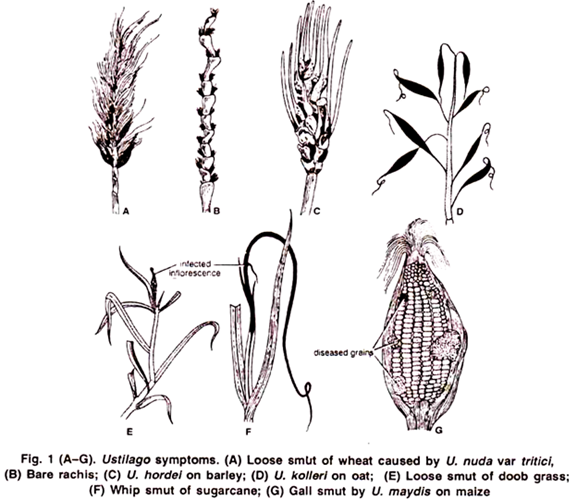
The symptoms appear only on the floral parts. The floral spikes turn black and remain filled with the smut spores.
Ustilago produces two main types of symptoms:
ADVERTISEMENTS:
1. The blackish powder of spores is easily blown away by the wind, leaving a bare stalk of inflorescence (Fig. 1 B). Species showing such symptoms are called loose smuts e.g.,
(a) Loose smut of oat caused by U. avenae
(b) Loose smut of barley caused by U. nuda
(c) Loose smut of wheat caused by U. nuda var. tritici. (Fig. 13A, B).
(d) Loose smut of doob grass caused by U. cynodontis (Fig. 1 E).
2. The blackish powder of spores remains covered by the wall of the grain (peridium), and the spores are liberated only by the breaking of wall during thrashing. Species showing such symptoms are called covered smuts e.g.,
Covered smut of Barley caused by U. hordei. (Fig. 1 C)
Covered smut of oat caused by U. kolleri. (Fig. 1 D)
ADVERTISEMENTS:
U. maxdis is known as gall forming smut. In it large sized galls are formed on stalk, leaves or on the ear Fig. 1 G). In sugarcane the entire inflorescence is transformed into a several feet long black, whip- like structure. This disease is called whip smut of sugarcane caused by U. scitaminae (Fig. 1 F).
3. Vegetative Structure of Ustilago:
The mycellium is branched, septate, hyaline, intercellular, with or without haustoria.
It is of two types:
(i) Primary Mycelium:
ADVERTISEMENTS:
It is monokaryotic (uninucleate) and formed by the germination of basidiospores. It is of very short duration.
(ii) Secondary Mycelium:
It is formed by the dikaryotisation of the primary mycelium. It is dikaryotic i bi-nucleate) and extends particularly through the entire life.
In most smuts the mycelium is scattered throughout the various parts of the host. It is said to be systemic. However, in some smuts (corn smut) it remains confined to certain parts of the host and is called localised.
4. Reproduction in Ustilago:
It is of two types:
ADVERTISEMENTS:
(1) Asexual Reproduction
(2) Sexual Reproduction
(1) Asexual Reproduction:
ADVERTISEMENTS:
It takes place by fragmentation, budding of basidiospores and formation of conidia. However, it is of rare occurrence.
(2) Sexual Reproduction:
Ustilago is autoecious i.e., it completes its life cycle on a single host. Sex organs are completely absent. It produces two kinds of spores during its life cycle i.e., Teliospores or teleutospores and basidiospores (Fig. 2, 5).
(1) Teleutospores:
These are also known as chlamydospores, smut spores or bi-nucleate brand spores. These are produced by the cells of the secondary mycelium (dikaryotic mycelium). The secondary mycelium becomes active at the flowering time of the host and forms a dense mass of hyphae within the host tissues. It is composed of numerous short dikaryotic cells (Fig. 2 A-C).
The protoplast of each bi-nucleate cell rounds off and the wall becomes gelatinized. At this stage, the protoplast secretes a thick wall around itself. It results in the formation of a smut spores (Fig. 2 D). By the time the spores reach towards maturity, the gelatinous matter disappears and the spores are separated from each other.
ADVERTISEMENTS:
Each smut spore is bi-nucleate globose, yellow to brown with spiny, reticulate or smooth wall (Fig. 2 E).
Its thick wall can be differentiated into two layers:
(1) The outer thick layer i.e., exine or exosporium and
(2) The inner thin layer i.e., inteine or endosporium (Fig. 2 E).
The smut spores are disseminated by wind, insects or water, only a small rachis persist on the infected ear after the total dispersal of the spores (Fig. 2 B).
Germination of Teliospore:
ADVERTISEMENTS:
Under favourable conditions (moisture and temperature) and falling on suitable substratum (like soil, twigs or leaves) smut spores germinate within a day. The exosporium ruptures and endosporium comes out in the form of a short promycelium or epibasidium (Fig. 2 G). Prior to germinatium the two nuclei (one two of ‘+’ strain and other of strain) in each teleutospore fuse to form a synkaryon (Fig. 2 F).
The diploid nucleus migrates into the promycelium and divides meiotically resulting in a row of four nuclei (i.e., two of ‘+’ strain and two of strain) (Fig. 2 H, I). Three transverse septa are laid down in the promycelium resulting in the formation of four uninucleate cells. Each uninucleate cell of the promycelium sprouts a bud laterally towards its upper end.
Each nucleus divides mitotically into two, one of which remains in the cell but the other migrates into the developing bud. These uninucleate buds are called basidiospores. Out of four basidiospores two are of + strain and two are of – strain. Like yeast the single basidiospore may produce a large number of secondary basidiospores or Conidia by budding (Fig. 2 J, K).
Germination Basidiospore:
A single basidiospore is thin walled, uninucleate and oval to round in shape. It germinates by producing a fine germ tube (infection tube or infection thread) either on the soil or on the young host plant (Fig. 2 L). The germ tube is haploid or monokaryotic. When it comes in contact with the opposite strain, it fuses to form a dikaryotic cell (plasmogamy) which later on develops into a dikaryotic mycelium (Fig. 2 M).
This process is called diplodization or dikaryotisation. This dikaryotic mycellium further produces the teliospores. The infection tube individually is unable to grow and parasitize in ovary unless two infection tubes of opposite strains fuse and form a dikaryotic mycelium.
Dikaryotisation (Diplodisation):
The process in which the primary or monokaryotic mycelium is converted into secondary or dikaryotic mycelium is called dikaryotisation or diplodisation.
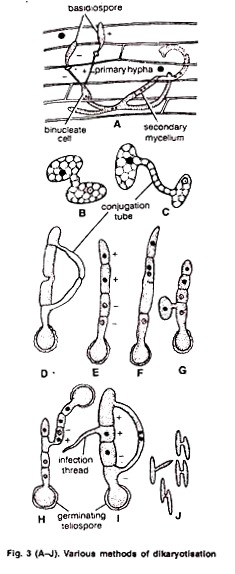
Dikaryotisation takes place by following methods:
1. By somatogamy (hyphal fusion) between primary mycelia. Two vegetative hyphae of opposite sexual strains fuse (Fig. 3 A).
2. By fusion between germ tubes of two germinating basidiospores. The germ tubes of the basidiospores of opposite strain meet and fuse (Fig. 3. B, C) e.g., U. avenae. Similarly, fusion between the two compatible cells of the epibasidium takes place by conjugation tube (Fig. 3 D) e.g., U. nuda.
3. By the Union of infection threads. Haploid cells of the promycelium grow into small, slender hyphae. These are called’ the infection threads. Two neighbouring infection threads of the opposite strain fuse e.g., U. tritici (Fig. 3 I).
4. By fusion between the two haploid cells of the same epibasidium. Fusion takes place between the two haploid cells of the same epibasidium e.g., U. hordei (Fig. 3 E, F).
5. By Fusion of basidial cell and basidiospore. A dikaryotic cell is formed between the fusion of a basidiospore with one of the basidial cells of the opposite strain (Fig. 3 G).
6. By the fusion of two basidia. A dikaryotic cell is formed by the fusion between two basidia formed by the germination of teleutospores of opposite strain e.g., U. nuda. (Fig. 3 H).
7. By Conjugation between the basidiospores. It occurs between sporidia of opposite mating types. Basidiospores produce secondary spores by budding. These are called sporidia. (Fig. 3 J).
8. Fusion of basidiospore of one strain with germ tube of the basidiospore of opposite strain.
Buller (1931) reported that if a monokaryon of Coprinus cinereus was opposed to a dikaryon it was possible for the homokaryon to be converted into monryotic state. It is known as Buller phenomenon.
Clamp Connection:
The secondary dikaryotic mycelium has frequently been found to produce structures called clamp connections. These are the characteristic features of Eubasidiomycetes and were first observed by Iloftman in 1856. Its development was first studied by kniep (1915) and Bensaudi (1918). Its function is probably to act as a bi-pass, to allow the migration of a nucleus from one cell to the other, which otherwise would not have been possible owing to the narrow lumen of hypha.
The clamp connections are formed as follows:
The two nuclei (+ and -) of the terminal cell of a mature dikaryotic hyphya divide simultaneously (conjugately) into four nuclei. (+, + and -, -). Simultaneously, a pouch like outgrowth arises from its wall (Fig. 4 A, B). One of the nuclei of the upper pair passes into the pouch and is cut off from the main cell by a septum at the base of the pouch. It may now be called a clamp cell (Fig. 4 C, D).
Simultaneously, one of the nuclei of the lower pair gets separated from its sister nucleus by a transverse wall (Fig. 4 E). At this stage, the terminal cell has two nuclei, the cell below it (penultimate cell) has one nucleus, while the fourth nucleus lies in the clamp cell. The clamp cell grows into hook like structure. Its tip bends over and finally fuses with the lateral wall.
The walls at the place of contact dissolve and the fourth nucleus passes into the penultimate cell (Fig. 4 F). Thus, the clamp cell serves as a by-pass or a bridge to transfer nucleus from one cell to the other and is known a clamp connection.
Control Measures of Smut Disease:
Since the mycelium is internally seed-borne, external application of fungicides normally remains ineffective.
The following methods are usually employed for its control:
1. Solar Heat-Treatment:
This method is applicable in those regions where the summer temperature is very high (42-44°C). It was suggested by Luthra and Sattar (1934).
Following two steps constitute this method:
(i) The seeds are soaked in water for at least four hours (8 am to 12 noon) on a bright summer day.
(ii) After this stage the seeds are dried in the sun for 4 hours from 12 noon to 4 p.m.
The germinating dormant mycelium is killed as a result of the action of the direct sun rays of hot summer.
2. Hot Water Treatment:
ADVERTISEMENTS:
This method was evolved by Jensin (1889) against late blight of potato. However, Swingle 1892. Freeman and Johnson (1909) used this method against loose smut of wheat.
It consists of two steps:
(i) Soaking the seeds in ordinary cold water (26-30°C) for four hours,
(ii) Dupping in hot water (54°C) for 10 minutes, and finally drying the grains before sowing. The first step reduces the dormant mycelium to germinate while the second step helps in killing the germ tubes.
(3) Anaerobic Seed Treatment. It consists of two steps:
(i) Soaking the seeds for 2-4 hours in water between 60-70°C.
(ii) Keermg the moist seeds in air tight containers for 65-70 hours and thereafter drying them.
(4) Growing Resistant Varieties:
It is the best method for avoiding the disease. Wheat varieties NP165, Pb9D. Bansi O.P., NP797, NP824, Kalyan 227, Pv 18, WG 307 and C 302 have been found to be resistant to this disease.
(5) Use of Fungicides:
In this method seeds are treated with Benomyl and Carboxin (vitavax, 0. 2 – 0.25%) or fungicides like Agrosan, spergon, ceresan, formaline and fine sulphur.
(6) Rogueing:
In this method the wheat plants with infected ears, which emerge out earlier than the healthy ones, are uprooted and burnt.
(7) Rotation of crops:
This method has also proved satisfactory in controlling this disease to some extent.