ADVERTISEMENTS:
In this article we will discuss about the reproduction in erysiphe. This will also help you to draw the structure and diagram of reproduction in erysiphe.
Asexual Reproduction in Erysiphe (Fig. 11.4):
With the establishment of the ectomycelium on the surface of the host, the fungal parasite starts reproducing asexually during the growing season by producing asexual spores known as conidia. The conidia are formed exogenously in chains at the tip of the conidial apparatus.
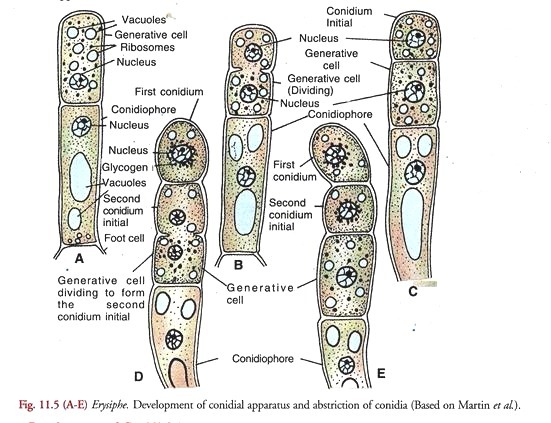
Development of Conidial Apparatus (Fig. 11.5):
According to Martin et al. (1983), it arises as an erect outgrowth from a cell in the older part of the superficial hypha. The outgrowth grows vertically approximately at right angle, to the surface of the host. It is known as the conidiophore initial.
The cell subtending the conidiophore initial is called the foot cell. A cross wall appears in the apical region of the elongated conidiophore initial dividing it into an upper conidiogenous or generative ceil and lower stalk or conidiophore cell. The latter in some species (E. graminis var. hordei) may be swollen at the base in a characteristic manner.
In a fully developed conidial apparatus, the conidiophore cell cytoplasm contains a few large vacuoles. The single nucleus is located in its upper part (A). It may as well contain glycogen granules.
ADVERTISEMENTS:
The conidiogenous or generative cell, on the other hand is filled with dense cytoplasm containing numerous ribosomes, small scattered vacuoles and no glycogen. It is uninucleate. The whole system borne on the foot cell and comprising the conidiophore, conidiogenous or generative cell and the conidium initials constitute the conidial apparatus.
Development of Conidia (Fig. 11.5 A-E):
The terminal generative cell undergoes division (B) and cuts off the first conidium initial at its tip (C). It is followed by the extension of the generative cell (D) and another division of the latter to produce the second conidium initial below the first which is pushed above (E). This periodic extension and division of the generative cell forms a succession of conidia which cling together in an upright chain (Fig. 11.4A).
The conidia in the chain represent successive stages in sequence of differentiation. They ripen from the apex downwards and then get abscised. The abscised conidia are readily dispersed by air currents. The disease spreads widely in summers by means of conidia which are produced in abundance in relatively cool, moist environment throughout most of the host’s growing season.
Conditions for germination of conidia:
The germination of powdery mildew conidia is dependent on temperature and relative humidity. They germinate over a wide range of temperature. The minimum is reported to be 0°C and maximum 30-36°C. The optimum is the range of 12- 25°C. Regarding humidity, neither free water nor high relative humidity is essential for their germination. In fact moistening of spores impairs germination.
ADVERTISEMENTS:
Their germination in dry atmospheric conditions is attributed to adequate internal water in the conidia. Nair and Ellingboe (1968) found that conidia of wheat powdery mildew showed highest germination at 17°C and 65 percent relative humidity. For the germination, refer to the heading “Primary Infection”.
Sexual Reproduction in Erysiphe (Fig. 11.6):
It takes place in summers. Excepting one species all others are homothallic. E. graminis lacks sexual branches altogether. In E. polygoni sex organs lie at the ends of sexual branches which arise near each other in pair from the somatic mycelium as lateral short erect uninucleate outgrowths. They lie closely parallel to and more or less twisted about each other.
(a) Antheridium:
ADVERTISEMENTS:
The nucleus of the male branch which is usually slender divides into two. The two nuclei are separated by a septum. The terminal uninucleate cell thus delimited functions as the anheridium and the lower supporting cell as the stalk cell.
(b) Ascogonium:
The female branch also consists of a terminal, uninucleate, slightly swollen, club-shaped ascogonium and the basal stalk cell.
ADVERTISEMENTS:
(c) Plasmogamy:
The antheridium makes contact with the ascogonium at its apex and closely presses against it. In the region of contact, the separating walls between the two sex organs break down and a pore is formed. The male nucleus passes into the ascogonium through the pore. A dikaryon is thus established in the ascogonium (B).
According to some investigators, fusion of male and female unclei takes place immediately in the ascogonium but this is denied by several others, who hold that the fusion of male and female nuclei is delayed until the ascus mother cell is formed.
ADVERTISEMENTS:
The consensus of opinion favours the latter view. At this stage sterile hyphae grow up from the stalk cell of the ascogonium and form a sheath around the sexual apparatus (C). The sheath or peridium is thick. It consists of three to many layers of compacted hyphae.
Post-plasmogamy changes:
The dikaryotised ascogonium elongates and divides by cross walls into a row of 4 or 5 cells (C). The penultimate cell or segment of the row contains two or more nuclei. Ascogenous hyphae bud out from this cell (D) and branch to form a dense interwoven mass of hyphae which develop slowly within the peridium through the winter.
Binucleate, intercalary cells of the ascogenous branches about 5 to 20 in number function as ascus mother cells. The two nuclei in the ascus mother cell fuse to form a synkaryon.
The young ascus with a synkaryon increases greatly in size. The fusion nucleus (synkaryon) in it undergoes meiosis followed by a single mitosis to form eight haploid nuclei. This is the octonucleate stage in the development of an ascus. In spring the asci in the cleistothecium mature to produce ascospores by free cell formation.
Depending upon the species the number of ascospores produced in each ascus after the octonucleate stage varies from two to eight. The remaining nuclei lie in the epiplasm and degenerate. The ascospores are not fully ripened until the following spring.
ADVERTISEMENTS:
Cleistothecium (Fig. 11.7):
The cleistothecia are globose structures without an opening. They appear as black specks on the surface of white mycelium. In some species, they are immersed in the mycelium. The peridium may consist of 6 to 10 cells in thickness. The component cells are polygonal in shape. The cells in the outer layers are without protoplast and are thick-walled.
Certain superficial cells of the peridium develop into long hypha-like, unbranched (A) or slightly branched appendages. The number of these appendages varies from 2 to 8 in E. polygoni but it goes up to 25 in E. graminis. All the asci originate from the base of the cleistothecial cavity, and spread out in a fan-shaped manner (Fig. 11.6 E).
They are ovate and almost sessile in E. polygoni but cylindrical or ovoid in some other species. The number of ascospores in each ascus varies from two to eight. In E. cichocearum there are only two rarely three ascospores in an ascus. The ascospores are hyaline, one-celled, unicellular structures elliptical in shape.
Dehiscence of Cleistothecium (Fig. 11.7 B):
ADVERTISEMENTS:
The mature cleistothecia remain dormant during winter. The thick peridium remains intact and enables them to resist adverse (winter) conditions. With the onset of spring, the epiplasm in the asci undergoes a change which results in increase in the osmotic pressure within them.
Consequently, the asci absorb water and swell. The swelling of the contents causes an irregular rupturing of the upper portion of the peridium (B). Salmon (1903) reported that in E. graminis, peridium splits in the equatorial plane by a transverse split. The upper half is shed and the asci protrude.
The exposed asci burst under increased internal pressure. The ascospores are shot out with a sufficient force which hurls them into the air to be dispersed. The liberated ascospore (C) germinates immediately on falling on a suitable host (D) to start new foci of infection.