ADVERTISEMENTS:
The following points highlight relation of various genera of fungi with mycotoxins. The genera are: 1. Mycotoxins of Aspergillus 2. Mycotoxins of Penicillium 3. Mycotoxins of Fusarium.
Genera # 1. Mycotoxins of Aspergillus:
The Aflatoxins. In 1959 a very singular event occurred which initiated the international interest which now exists in mycotoxins.
This was the deaths of several thousand turkey poults and other poultry on farms in East Anglia and, because of the implications for the turkey industry and the manufacture of pelletted feed which supported it, a considerable effort was put into understanding the etiology of this major outbreak of what was initially referred to as turkey X disease.
ADVERTISEMENTS:
Although the name implies a disease such as a viral infection, it was shown that the birds had been poisoned by a contaminant in the groundnut meal used as a protein supplement in the pelletted feed. The contaminant, which was called aflatoxin, fluoresces intensely under ultra-violet light and was shown to be produced by the mould Aspergillus flavus growing on the groundnuts.
Aflatoxin is not only acutely toxic but, for the rat, it is amongst the most carcinogenic compounds known. The demonstration of the potential carcinogenicity of aflatoxin made it possible to rationalize the etiology of diseases such as liver carcinoma in rainbow trout and hepatitis X in dogs which had been described nearly a decade earlier but had remained a mystery.
Very sensitive analytical methods for aflatoxins were developed which led to the demonstration that their occurrence was widespread in many agricultural commodities, especially groundnuts and maize, much of which may be destined for human consumption.
The aflatoxins are now known to be produced by two closely related species of mould, Aspergillus flavus and A. parasiticus both of which are especially common in the tropics and subtropics.
ADVERTISEMENTS:
More recently a third species, A. nomius, has been recognized as aflatoxigenic, but the frequent reports in the early literature of the production of aflatoxins by other species, even belonging to different genera, are usually the result of artefacts or mistakes.
Initially, it was considered that aflatoxin contamination was essentially a problem of poor storage of commodities after harvest allowing the growth of storage fungi such as aspergilli and penicillia with consequent formation of mycotoxins.
Indeed, conditions of high humidity and warm temperatures can give rise to the highest levels of aflatoxin in food often exceeding the upper limit initially established by the Food and Agricultural Organization (FAO) and the World Health Organization (WHO) of 30 µg kg– 1 in foods for human consumption.
It has to be recognized that these agencies faced a hard dilemma when setting these limits and this is reflected in the observation that ‘clearly the group would have preferred a lower figure, but felt that the danger of malnutrition was greater than the danger that aflatoxin would produce liver cancer in man’.
Meanwhile, many developed countries had set even more stringent legislative or guideline levels, some of the more recent of which are shown in Table 8.2.
Like many microbial secondary metabolites, the aflatoxins are a family of closely related compounds, the most toxic of which is referred to as aflatoxin B1 (Figure 8.9). The precise nature of the response to aflatoxin is dependent on species, sex and age.
Some animals, such as the day-old duckling and the adult dog, are remarkably sensitive to the acute toxicity of aflatoxin B1 with LD50 values of 0.35 and 0.5 mg kg– 1 body weight respectively, while others, such as the adult rat and the mouse, are more resistant (LD50 ca. 9 mg kg-1).
ADVERTISEMENTS:
Not all animals respond to the carcinogenic activity of aflatoxin but for the rat and the rainbow trout aflatoxin B1 is one of the most carcinogenic compounds known.
What about humans? Is man as sensitive as the dog or as resistant as the rat to the acute toxicity and does aflatoxin cause liver cancer in man?
A particularly tragic demonstration of the acute toxicity of aflatoxin to man was reported in India in 1974 when a large outbreak of poisoning occurred involving nearly 1000 people of whom nearly 100 died. From the concentrations of aflatoxins analysed in the incriminated mouldy maize it is possible to estimate that the LD50 of aflatoxin B1 in man lies somewhere between that for the dog and the rat.
ADVERTISEMENTS:
Although aflatoxin may be considered amongst the most carcinogenic of natural products for some animals, it is still not clear whether it is a carcinogen for man. Liver cancer in some parts of the world, such as the African continent, is complex and the initial demonstration of a correlation between exposure to aflatoxin in the diet and the incidence of liver cancer has to be considered with caution.
It is known that a strong correlation occurs between the presence of hepatitis B virus and primary liver cancer in humans and there is an increasing consensus that these two agents act synergistically.
Although liver cancer may be attributable to exposure to aflatoxin in parts of Africa it is necessary to ask why liver cancer is not also more prevalent in India where dietary exposure to aflatoxin also occurs. In India, cirrhosis of the liver is more common and there is still a lot to learn about the role of aflatoxin in liver cancer and liver damage in different parts of the world.
A diverse range of responses to the toxic effects of a compound may occur because the compound is metabolized in the animal body and the resulting toxicity is influenced by this metabolic activity. This is certainly the case with aflatoxin B1 from which a very wide range of metabolites are formed in the livers of different animal species (Figure 8.10).
ADVERTISEMENTS:
Thus the cow is able to hydroxylate the molecule and secrete the resulting aflatoxin Ml in the milk, hence affording a route for the contamination of milk and milk products in human foods even though these products have not been moulded.
The formation of an epoxide could well be the key to both acute and chronic toxicity and those animals which fail to produce it are relatively resistant to both. Those animals which produce the epoxide, but do not effectively metabolize it further, may be at the highest risk to the carcinogenic activity of aflatoxin B1 because the epoxide is known to react with DNA.
Those animals which not only produce the epoxide but effectively remove it with a hydrolase enzyme, thus producing a very reactive hydroxy-acetal, are most sensitive to the acute toxicity. The hydroxy-acetal is known to react with proteins.
ADVERTISEMENTS:
The parent molecule may thus be seen as a very effective delivery system having the right properties for absorption from the gut and transmission to the liver and other organs of the body. It is, however, the manner in which the parent molecule is subsequently metabolized in vivo which determines the precise nature of an animal’s response.
Information available about the metabolic activity in the human liver suggests that man is going to be intermediate in sensitivity to the acute toxicity and may show some sensitivity to the chronic toxicity of aflatoxin B1, including carcinogenicity.
Several studies have demonstrated that very young children may be exposed to aflatoxins even before they are weaned because mothers, consuming aflatoxin in their food, may secrete aflatoxin M1 in their milk. There is no doubt about the potential danger of aflatoxin in food and ever)’ effort should be made to reduce or, if possible, eliminate” contamination.
The Ochratoxins:
Ochratoxin A (Figure 8.11), which is a potent nephrotoxin, was first isolated from Aspergillus ochraceus in South Africa, but it has been most extensively studied as a contaminant of cereals, such as barley, infected with Penicillium verrucosum in temperate countries such as those of northern Europe.
This is because it is known to be a major aetiological agent in kidney disease in pigs and, because it is relatively stable, ochratoxin A may be passed through the food chain in meat products to man.
A debilitating disease in man known as Balkan endemic nephropathy, the epidemiology of which is still a mystery, may be associated with the presence of low levels of nephrotoxic mycotoxins, such as ochratoxin, in the diet of people who have a tradition of storing mould-ripened hams for long periods of time.
The presence of ochratoxin in foods of tropical and subtropical origin, such as maize, coffee beans, cocoa and soya beans is usually due to contamination by Aspergillus species.
Other Aspergillus Toxins:
Sterigmatocystin (Figure 8.12), a precursor in the biosynthesis of aflatoxins, is produced by a relatively large number of moulds but especially by Aspergillus versicolor.
It is not considered to be as acutely toxic, or as carcinogenic, as aflatoxin but it is likely to be quite widespread in the environment and has been isolated from a number of human foods such as cheeses of the Edam and Gouda type which are stored in warehouses for a long period of time.
In this situation the moulds grow only on the surface and sterigmatocystin does not penetrate beyond the first few millimeters below the surface.
Cyclopiazonic acid (Figure 8.13) gets its name because it was first isolated from a mould which used to be called Penicillium cyclopium (now known as P. aurantiogriseum) but it has subsequently been isolated from Aspergillus versicolor and A. flavus.
In the latter, it is formed primarily in the sclerotia and there has always been a suspicion that some of the symptoms ascribed to the ingestion of food contaminated by A. flavus may be due to the presence of this compound as well as to the presence of aflatoxins.
In parts of India a disease known as kodua poisoning occurs following the consumption of kodo millet (Paspalum scrobiculatum) which is both a staple food and an animal feed. Aspergillus flavus and A. tamarii have been isolated from incriminated samples of millet and both species are able to synthesize cyclopiazonic acid.
Poisoning in cattle and humans is associated with symptoms of nervousness, lack of muscle co-ordination, staggering gait, depression and spasms and, in humans, sleepiness, tremors and giddiness may last for one to three days.
Some of these symptoms are reminiscent of a problem in intensively reared farm animals known as staggers in which complex indole alkaloid metabolites (tremorgens) are implicated. One of these metabolites, aflatrem, is also produced by some strains of A. flavus.
Genera # 2. Mycotoxins of Penicillium:
Penicillium is much more common as a spoilage mould in Europe than Aspergillus with species such as P. italicum and P. digitatum causing blue and green mould respectively of oranges, lemons and grapefruits, P. expansum causing a soft rot of apples, and several other species associated with the moulding of jams, bread and cakes.
Species which have a long association with mould-ripened foods include P. roquefortii and P. camembertii, used in the mould ripened blue and soft cheeses respectively.
The mycotoxin patulin (Figure 8.14) is produced by several species of Penicillium, Aspergillus and Byssochlamys but is especially associated with P. expansum and was first described in 1942 as a potentially useful antibiotic with a wide spectrum of antimicrobial activity.
It was discovered several times during screening programmes for novel antibiotics and this is reflected in the many names by which it is known including claviformin, clavicin, expansin, penicidin, mycoin, leucopin, tercinin and clavatin.
It was not until 1959 that an outbreak of poisoning of cattle, being fed on an emergency ration of germinated barley malt sprouts, alerted the veterinary profession to patulin as a mycotoxin.
In this instance the producing organism was Aspergillus clavatus but the same toxin has been implicated in several outbreaks of poisoning from such diverse materials as apples infected with P. expansum to badly stored silage infected with a species of Byssochlamys.
As far as humans are concerned, it is the common association of P. expansum with apples, and the increasing consumption of fresh apple juice as a beverage, which has caused some concern. Patulin is not a particularly stable metabolite, but it is stable at the relatively low pH of apple juices, although it is destroyed during the fermentation of apple juice to cider.
Even if there was no concern over the toxicity of patulin, the demonstration of its presence in a fruit juice is a useful indicator that very poor quality fruit has been used in its manufacture.
The nephrotoxic metabolite citrinin, produced by P. citrinum, was also first discovered as a potentially useful antibiotic but again rejected because of its toxicity. It is probably not as important as ochratoxin, produced by P. verrucosum as well as Aspergillus ochraceus, although it may be implicated in the complex epidemiology of ‘yellow rice disease’
Yellow Rice Disease:
A complex of disorders recognized in Japan a number of times since the end of the last century has been associated with the presence of several species of penicillia and their toxic metabolites on rice. This moulded rice is usually discoloured yellow and several of the toxic metabolites implicated are themselves yellow pigments.
There was an early awareness that moulds may be responsible for cardiac beriberi and in 1938 it was demonstrated that Penicillium citreo-viride (P. toxicarium) and its metabolite citreoviridin were responsible.
The most toxic of the species of penicillia associated with yellow rice disease is P. islandicum which produces two groups of toxins, hepatotoxic chlorinated cyclopeptides such as islanditoxin, as well as the much less acutely toxic, but potentially carcinogenic, di-anthraquinones such as luteoskyrin.
Genera # 3. Mycotoxins of Fusarium:
Some species of Fusarium cause economically devastating diseases of crop plants such as wilts, blights, root rots and cankers, and may also be involved in the post-harvest spoilage of crops in storage. The genus is also associated with the production of a large number of chemically diverse mycotoxins.
Alimentary Toxic Aleukia:
Outbreaks of this dreadful disease, which is also known as septic angina and acute myelotoxicosis, occurred during famine conditions in a large area of Russia. A particularly severe outbreak occurred during the period 1942-47 but there had been reports of the disease in Russia since the 19th Century.
Studies in Russia itself demonstrated that the disease was associated with the consumption of cereals moulded by Fusarium sporotrichioides and F. poae but the nature of the toxin remained unknown. Studies of dermonecrosis in cattle in the United States were shown to be caused by a Fusarium metabolite called T-2 toxin (Figure 8.15) which is one of the most acutely toxic of a family of compounds called trichothecenes.
There is good evidence that T-2 toxin was a major agent in the development of alimentary toxic aleukia in man, the first symptoms of which are associated with damage of the mucosal membranes of mouth, throat and stomach followed by inflammation of the intestinal mucosa.
Bleeding, vomiting and diarrhoea, which are all associated with damage of mucosal membrane systems, were common but recovery at this stage was possible if the patient was given a healthy, uncontaminated, vitamin-rich diet.
Continued exposure to the toxin, however, led to damage of the bone marrow and the haematopoietic system followed by anaemia and a decrease in erythrocyte and platelet counts. The occurrence of necrotic tissue and skin haemorrhages were further characteristics of the disease.
As well as giving rise to this sequence of acute symptoms, the trichothecenes are known to be immunosuppressive and this undoubtedly contributed to victims’ sensitivity to relatively trivial infectious agents. Indeed, many people died of bacterial and viral infections before succumbing from the direct effects of the toxin itself.
Unlike aflatoxin, the acute toxicity of T-2 toxin is remarkably uniform over a wide range of animal species (Table 8.3) and it is reasonable to assume that the human LD50 will be in the same range. Although improved harvesting and storage has eliminated alimentary toxic aleukia from Russia this disease may still occur in any part of the world ravaged by war and famine.
Three of the most important mycotoxins, aflatoxin, ochratoxin and T-2 toxin, are immunosuppressive but react differently against the immune system. All three inhibit protein biosynthesis, aflatoxin by inhibiting transcription, ochratoxin by inhibiting phenylalanyl tRNA synthetase, and T-2 toxin by inhibiting translation through binding with a specific site on the eukaryote ribosome.
One consequence of these distinct modes of activity is that mixtures of such mycotoxins are likely to be synergistic in activity and this has been shown experimentally in the case of aflatoxin and T-2 toxin. This observation is significant in the context of the probability that a food which has gone mouldy will probably be infected by several species of mould and may thus be contaminated by several different mycotoxins.
DON and Other Trichothecenes:
In Japan an illness known as red-mould disease involving nausea, vomiting and diarrhoea has been associated with the consumption of wheat, barley, oats, rye and rice contaminated by species of Fusarium.
The species most frequently incriminated was Fusarium graminearum, although it had been misidentified as F. nivale, and the trichothecene toxins isolated from them were called nivalenol and de-oxy-nivalenol. It is now realized that F. nivale itself does not produce trichothecenes at all, indeed it may not even be a Fusarium.
De-oxy-nivalenol (Figure 8.15), also known as DON and vomitoxin, was also shown to be the vomiting factor and possible feed-refusal factor in an outbreak of poisoning of pigs fed on moulded cereals in the United States. De-oxy-nivalenol is much less acutely toxic, than T-2 toxin, having an LD5o of 700 mg kg – 1 in the mouse.
Nevertheless, it is more common than T-2 toxin especially in crops such as winter wheat and winter barley. In 1980 there was a 30-70% reduction in the yields of spring wheat harvested in the Atlantic provinces of Canada due to infections with Fusarium graminearum and F. culmorum, both of which may produce DON and zearalenone.
It is not clear whether DON and other trichothecenes are as immunosuppressive as T-2 toxin but it seems prudent to reduce exposure to a minimum.
The most virulent group of trichothecenes are those with a macro cyclic structure attached to the trichothecene nucleus such as the satratoxins, verrucarins and roridins produced by Stachybotrys atra (Figure 8.15). This species has been implicated in a serious disease of horses, referred to as stachybotryotoxicosis, fed on mouldy hay. It seems that species of Fusarium do not produce such toxins.
Zearalenone:
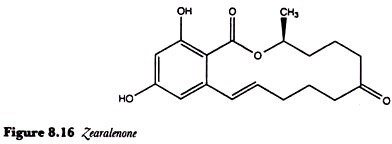
Zearalenone (Figure 8.16) is an oestrogenic mycotoxin which was first shown to cause vulvovaginitis in pigs fed on mouldy maize. Pigs are especially sensitive to this toxin and, although its acute toxicity is very low, it is common in cereals such as maize, wheat and barley being produced by Fusarium graminearum, F. culmorum and other species of Fusarium.
The toxin was called zearalenone because of its initial isolation from Gibberella zeae, the perfect stage of F. graminearum.
In gilts, the vulva and mammary glands become swollen and, in severe cases, there maybe vaginal and rectal prolapse. In older animals there may be infertility, reduced litter size and piglets may be born weakened or even deformed. There is concern about the long-term exposure of the human population to such an oestrogen.
Zearalenone, and the corresponding alcohol zearalenol, are known to have anabolic, or growth promoting activity, and, although its use as a growth promoting agent is banned in some countries, it is permitted in others. This can lead to problems in international trade because zearalenone can be detected in the meat of animals fed on diets containing it.
Oesophageal Cancer:
In parts of Northern China, and the Transkei in Southern Africa, there are regions of high incidence of human oesophageal carcinoma and the epidemiology of the disease fits the hypothesis that the consumption of moulded cereals and mycotoxins are involved. F. moniliforme, which belongs to a distinct group of the genus which do not produce trichothecenes, seems to be the most likely fungus to be involved.
Strains of this species are associated with a disease of rice which has been a particular problem in China and other, probably distinct, strains are commonly isolated from maize grown in Southern Africa. F. moniliforme is a very toxigenic species and its occurrence in animal feeds is associated with outbreaks of a disease known as equine leukoencephalomalacia in horses and liver cancer in rats.
One of the first mycotoxins to be isolated during the study of these diseases was called moniliformin (Figure 8.17) because it was presumed to have been produced by F. moniliforme. It is now known that moniliformin is actually produced by strains of the related species F. subglutinans and not F. moniliforme.
However, it is the latter which is especially associated with human oesophageal cancer and a number of complex metabolites have been isolated and characterized from cultures of this species, including fusarin C, which is mutagenic, and the fumonisins which are carcinogenic (Figure 8.18).
However it would probably be wise to be cautious about extrapolating laboratory tests demonstrating carcinogenic activity to a human disease and it is probable that there is still more to learn about this important mould.
Mycotoxins of Other Fungi:
Ergotism has been documented as a human disease since the middle ages but its aetiology remained a mystery until the mid-19th Century when it was demonstrated to be caused by a fungus, Claviceps purpurea.
This fungus is a specialized parasite of some grasses including cereals and, as part of its life cycle, the tissues of infected grains are replaced by fungal mycelium to produce a tough purple brown sclerotium which is also known as an ergot because it looks like the spur of a cockerel (Figure 8,19).
The biological function of the sclerotium is to survive the adverse conditions through the winter in order to germinate in the following spring. Ergots contain alkaloid metabolites which may be incorporated into the flour, and eventually the bread, made from the harvested grain.
Ergotism, or St Anthony’s fire, is infrequent in human beings. The toxicity of the ergot alkaloids is now well understood and one aspect of their activity is to cause a constriction of the peripheral blood capillaries leading, in extreme cases, to fingers and toes becoming gangrenous and necrotic.
Different members of this family of mould metabolites may also have profound effects on the central nervous system stimulating smooth muscle activity.
Plant-fungal interactions can be complex and there are instances where a toxic plant metabolite is produced in response to fungal attack. Thus, when the sweet potato, Ipomoea, is damaged by certain plant pathogens it responds by producing the phytoalexin ipomearanone.
This antifungal agent is produced to limit fungal attack but it is also an hepatotoxin to mammals. Further complexity arises when other moulds, such as Fusarium solani, degrade ipomeamarone to smaller molecules such as ipomenol which can cause oedema of the lung (Figure 8.20).
A disease of sheep in New Zealand known as ryegrass staggers may cause an estimated loss of hundreds of millions of dollars in some years. It is caused by an intimate association of perennial ryegrass (Lolium perenne) and an endophytic fungus, Acremonium loliae.
The endophyte-plant association results in the production of complex tremor genic mycotoxins known as the lolitrems (Figure 8.21) which are responsible for the staggering response and possible collapse of sheep under stress. The endophyte is seedborne and completes its whole life cycle within the plant, although it can be cultured with difficulty in the laboratory.
It is possible to eliminate the endophyte by careful heat treatment of seed but, in New Zealand, the planting of endophyte-free ryegrass provides pastures which are very susceptible to insect damage such as that caused by the stem weevil Listronotus bonariensis.
It is almost certain that the role of the endophyte in controlling insect damage is not due to the production of lolitrems so the possibility remains that a genetically engineered strain of Acremonium loliae, which no longer produces lolitrems, could be used to replace the wild strain in perennial ryegrass.
Although there can be no doubt about the potential for the presence of mycotoxins in food to cause illness and even death in man, there are far more overt mycotoxicoses in farm animals throughout the world.
Thus facial eczema in sheep in New Zealand, caused by the saprophyte Pithomyces chartarum growing on dead grass, slobbers in cattle in the United States, caused by Rhizoctonia leguminicola parasitic on red clover, and lupinosis of farm animals in Australia, caused by Phomopsis leptostromiformis growing on lupins, do not have a direct effect on humans.
However there can be no denying the impact that such outbreaks of mycotoxin poisoning have on economics through losses in productivity.
Recognition of the potential to cause harm in humans, by the imposition of maximum tolerated levels of mycotoxins such as aflatoxin, can also have a major impact on economics by rendering a commodity unacceptable in national or international trade. Thus, a major problem occurred for Turkey, the world’s most important exporter of dried figs, during the Christmas of 1988.
Several European countries imposed a ban on the import and sale of dried figs following the demonstration of aflatoxin in 30% of samples of figs analysed. Fearing the loss of 50 000 jobs in the fig-drying and packing industry, Turkey was vigorous in her diplomatic efforts to have the bans lifted.
This was done fairly soon after they had been imposed and an international symposium on ‘Dried Figs and Aflatoxins’ was held in Izmir, Turkey, in April 1989.
In 1980, nearly 66% of random samples of maize from North Carolina had concentrations of aflatoxins in excess of 20 µg kg -1 giving rise to an estimated loss to producers and handlers of nearly 31 million dollars. It is rare that the losses and costs arising from mycotoxin contamination can be calculated but these two isolated and very different examples indicate that on a world-wide basis they must be considerable.
In both these examples aflatoxin was probably formed in the commodity during growth and development in the field. Under these conditions aflatoxin formation is usually relatively low and in neither case was there any evidence of harm to human beings.
However, it is when commodities are improperly stored that really high concentrations of mycotoxins may be formed and it is in these situations that human suffering can occur.