ADVERTISEMENTS:
In this article we will discuss about the citric acid production by various microorganisms. Learn about:- 1. Production of Citric Acid by Fermentation 2. Citric Acid Production 3. Commercial Processes for Citric Acid Production 4. Citric Acid Production by Aspergillus Niger 5. Submerged Fermentation of Citric Acid.
Citric Acid Production by Fungi:
Fungal Strains:
Since the early demonstration by Wehmer (1893) of the presence of citric acid in culture media containing sugars and inorganic salts with species of Penicillium, a variety of fungi have been screened for citric acid production. The various fungi which have been found to accumulate citric acid in their culture media include strains of Aspergillus niger, A. awamori, A. fonsecaeus, A. luchensis, A. wentii, A. saitoi, A. usami, A. fumaricus, A. phoenicus, A. lanosus, A. flavus, Penicillium janthinellurn, P. restrictum, Trichoderma viride, Mucor piriformis, Ustulina vulgaris, and species of Botrytis, Ascochyta, Absidia, Talaromyces, Acremonium, and Eupenicillium.
ADVERTISEMENTS:
Currie (1917) first reported that strains of A. niger, when cultured in a sugar-containing medium of low pH, produced citric acid. Prior to that, this organism was known to produce only oxalic acid. In fact, even today, this organism when cultured in sugar-containing media at a higher pH produces oxalic acid.
Since this finding, strains of A. niger have dominated others both in laboratory and industrial scale production of citric acid. The major advantages in using this organism for producing citric acid are- (1) the ease with which it can be handled, (2) the cheap raw materials that it can utilize for citric acid production, and (3) high and consistent yields, thereby making the process economical.
ADVERTISEMENTS:
Types of Fermentations:
The development of processes for citric acid fermentation can be divided into 3 phases. In the first phase, citric acid production was confined to species of Penicillium and Aspergillus under stationary or surface culture conditions. The second phase, beginning in the 1930s, consisted of the development of submerged fermentation processes for citric acid production using A. niger.
The third stage, which is of recent origin, involves the development of solid state culture, continuous culture, and multi-stage fermentation techniques for citric acid production. Thus, there are at least 3 principal methods which are today available for producing citric acid using fungi.
In the surface culture technique, sterile nutrient medium containing sugar is allowed to flow into stainless steel or high grade aluminum trays which are arranged in tiers in sterile fermentation chambers. The size and the number of trays and the volume per tray vary from plant to plant.
Most fermentation chambers have controls over temperature, relative humidity, and circulation of purified air. The fermentation medium is then inoculated with spores of A. niger and the temperature is maintained around 28°-30°C and the relative humidity between 40 and 60% for 8-12 days.
As the organism grows and spreads over the surface, the medium becomes acidic and the course of fermentation can be followed by determining either the pH or the total acid content of the broth. After the fermentation is finished, the fermented liquor is drained off and further processed for recovery of citric acid. In some cases, the preformed mycelium is reused for one or two more rounds of fermentation. The fermentation chambers and trays are then sterilized using water, dilute formaldehyde, and sulfur dioxide.
Although the surface process is still being used, most of the newly built plants have adopted the submerged or the deep fermentation process. In this, the nutrient media after inoculation are subjected to vigorous, controlled aeration and agitation in large fermentors. The time interval involved is much shorter (3-5 days) at temperatures of 25°-30°C. The mother liquor after fermentation is drained off and citric acid is extracted. As in the surface culture, sometimes the mycelial pellets are reused for a second fermentation.
A 2-stage submerged fermentation process involving a “growth stage” and a “production stage” has also been developed. In this, the growth medium is first inoculated with the spores and after 3-4 days of growth, the mycelium is separated from the solution and added to the fermentation medium. The fermentation is then carried out for 3-4 days at 25°-30°C while oxygen is dispersed through the medium.
The third process, namely, the solid state fermentation, was first described by Cahn (1935). Despite its potential, this technique has not drawn much attention mainly because it is a labor intensive technique. In this process, the fermentation medium is impregnated in porous solid materials such as sugarcane bagasse, potato or beet pulp, pineapple pulp, etc., in an appropriate ratio, sterilized, and then inoculated with a suspension of fungal spores.
ADVERTISEMENTS:
The mash is then incubated in trays at 25°-30°C for 6-7 days. The height of the mash during incubation can be variable. After fermentation, the mash is extracted with water, concentrated, and then processed for citric acid precipitation.
In recent years semi-continuous, continuous, or multi-stage processes have been patented for citric acid production. However, full details of these processes are not available. Zhuravskii et al. (1974) have patented a continuous multi-stage process for citric acid production. In this process culture medium is added at a rate providing for its complete replacement in 24 hr.
The inflow coefficient of the medium is kept at 4%. The medium from the first stage then flows to a second fermentor. It is then aerated gradually with increasing amounts of air from 20 to 30 m3/m3/hr. During the fermentation, caustic alkali is also added in such a way that one-third of the citric acid formed is neutralized.
In another Russian patent, Brehznoi et al. (1976) have described a continuous process for citric acid production. In this, the nutrient medium containing molasses is added at a constant rate and the final product is removed at a rate that maintains sugar concentration and total acidity at a constant level.
ADVERTISEMENTS:
The semi-continuous processes described in literature or patents are mostly replacement culture processes. In these, after the fermentation under batch culture condition is over, the mother liquor is withdrawn and replaced with fresh sterile medium. This process has been partially successful and makes use of the fact that citric acid production occurs by cells which are not in the active stages of growth. Thus, the use of mycelia for subsequent fermentation, although it does not represent a continuous process in the correct sense, is yet close to a semi-continuous culture technique. Although this process is economical, subsequent yields are generally lower.
Despite various advances in process development, the surface and submerged batch culture techniques are still being used as the exclusive processes for large-scale production of citric acid. Depending upon the strain of fungus, appropriate cultural conditions have to be developed.
ADVERTISEMENTS:
Cultural Conditions:
Carbon, Nitrogen, and Phosphate:
ADVERTISEMENTS:
Cultural conditions for citric acid production by fungi vary from strain to strain and also depend on the type of process. This has been so since a strain that produces citric acid efficiently under surface culture conditions fails to do so under submerged culture conditions. Also, strains that can use one carbon source efficiently fail to show good acid production when cultured in a medium containing another. Because of these difficulties, the best substrate for each organism has to be determined.
During the early periods of citric acid fermentation with Penicillium species, culture media contained glucose in the range of 10-12% concentration with incubation times of 4-6 weeks at 15°-20°C. Under these conditions about 50% of the glucose was converted into citric acid.
Efficient citric acid production by fungi requires simple synthetic media. In fact, it has been found that yields are much higher when fungi are cultivated in simple synthetic media than in complex media. This may be due to the influence of metals and other components in citric acid production.
These days a variety of carbon sources, such as sucrose, citrus molasses, cane juice, starch from various sources, cane and beet molasses, have been used for citric acid production, yet sucrose and molasses have remained mostly the substrates of choice. The use of beet or cane molasses has made the process economical although yields may be slightly low.
The initial sugar concentration has been found to determine the amount of citric acid and also the amount of other organic acids produced by A. niger. Normally, strains of A. niger need a fairly high initial concentration (15-18%) of sugars in the medium. A concentration higher than 15-18%, however, leads to greater amounts of residual sugars, making the process uneconomical; while on the other hand, a lower concentration of sugar leads to lower yields of citric acid as well as to the accumulation of oxalic acid.
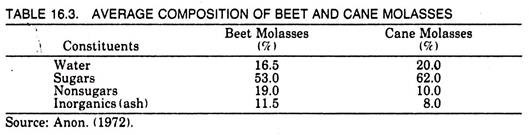
Molasses, a by-product of the sugar industry, is the syrupy liquid left after the removal of sugar from the mother syrup. It contains 50-60% sucrose which cannot be removed by simple crystallization. The average composition of molasses is given in Table 16.3.
There are three types of molasses, the blackstrap, refinery, and invert or high test molasses. The blackstrap molasses is molasses from the sugar factory obtained from the last stages of crystallization while refinery molasses is that molasses obtained at a second stage of refining sugar. Invert or high test molasses is partly inverted cane juice syrup from which no sugar has been extracted.
Refinery molasses contains about 48-50% sugar and has relatively less ash content. The sucrose contained in molasses is well suited as a raw material for fermentation processes. However, one major disadvantage in using this material for citric acid production is its high ash content, which inhibits efficient citric acid production.
When molasses is used, it is diluted to a sugar concentration of 15-20% with dilute sulfuric acid and to a pH 5.5-6.5. Other nutrients required for fungal growth are then added and the mixture is sterilized for 30 min. To reduce or eliminate the inhibitory action of metals, appropriate pretreatment is given to the molasses solution.
Citric acid production by A. niger involves both a “growth stage” and a “fermentation stage.” The organisms therefore need major elements such as carbon, nitrogen, phosphorus, and sulfur in addition to various trace elements for growth and citric acid production.
The concentration of all these constituents has a profound effect on the yield of citric acid. The nitrogen requirement for citric acid production is generally met by the addition of inorganic nitrogen sources such as (NH4)2SO4, NH4NO3, NaNO3, KNO3, urea, etc. However, the type of nitrogen source and its concentration affect the performance of the fungus considerably.
For example, ammonium sulfate prolongs vegetative growth while ammonium nitrate favors a shorter period of vegetative growth. However, a concentration of ammonium nitrate greater than 0.25% leads to the accumulation of oxalic acid.
ADVERTISEMENTS:
Among the nitrates, sodium and potassium nitrates have been found to be superior to ammonium nitrate for citric acid production by A. niger. Dhankar et al. (1974) found that sodium nitrate at a concentration of 0.4% was superior to ammonium nitrate. In general, a high concentration of nitrogen leads to greater vegetative growth and delays the onset of the production phase. It is, therefore, necessary to correctly determine the nitrogen source and the concentration essential for maximal citric acid production by different fungal strains under different fermentation conditions.
In addition to carbon and nitrogen, phosphate constitutes the third major essential element for fungal growth. The concentration of phosphate in the fermentation medium has a profound effect on the amount of citric acid produced. A high concentration of phosphate promotes more growth and less acid production.
Earlier reports suggested that citric -acid production begins only after the available phosphorus compounds are assimilated by the mold. Although there are not too many reports on this aspect, in general a phosphate concentration of about 0.1-0.2% in the fermentation medium appears to be adequate.
Trace Elements:
A. niger needs a variety of divalent trace elements such as Fe++, Cu++, Zn++, Mn++, and Mg++, etc., for growth and citric acid production. However, citric acid production is very sensitive to the concentration of these metals in the fermentation media. In fact, successful citric acid production depends to a great extent on the control of the concentration of trace elements.
Magnesium is essential for a variety of enzyme reactions in the cell and is required both for growth as well as for citric acid production. The optimum concentration of MgSO4 for maximum citric acid production varies from 0.02 to 0.025%.
Among the other trace elements, Fe++ and Zn++ have a critical role to play in determining the efficiency of fermentation. There are conflicting reports regarding the exact concentration of these two metals essential for optimal citric acid production, although it is generally agreed that the concentration should be very low. A high concentration of these metals allows vegetative growth at the cost of acid excretion.
The optimum concentration of Fe++ required for maximal citric acid production has been found to vary with the strain of fungus. Schweiger (1961) has reported that citric acid production by A. niger under submerged fermentation conditions using molasses as the substrate is severely affected by the presence of iron at a concentration as low as 0.2 ppm; however, the addition of copper at 0.1-500 ppm at the time of inoculation or during the first 50 hr of fermentation was found to counteract the deleterious effect of iron.
Such beneficial effect of Cu++ in counteracting the Fe++ effect has also been reported by Fedoseev et al. (1970), who found that the addition of CuSO4 at 4.7 mg/100 g molasses resulted in better conversion of sugar into citric acid.
The effect of Zn++ concentrations on citric acid production by A. niger has been a subject of much investigation. Like Fe++, the concentration of this metal drastically influences the outcome of the fermentation. Zn++ at a concentration of 1 – 2 μM allows continuation of the growth phase, but at a concentration of less than 1 μM restricts growth.
In fact, addition of excess of Zn++ to a citric acid-producing culture has been found to reverse the acid production phase. It is suggested that Zn++ has an important role in the regulation of growth and citric acid production and that Zn++ deficiency during growth apparently signals the transition from the growth phase to the acid production phase.
It has also been suggested that Zn++ has an indirect role in the functioning of cyclic AMP. While addition of cAMP during the production phase enhances citric acid production, addition of Zn++ retards acid production.
Other trace elements such as Mn++, Ba++, Al+++, etc., have been reported to have an effect on fungal morphology and citric acid production, at concentrations that generally do not inhibit growth. The exact role of some of these metals in citric acid production is not known.
Pretreatment of Raw Materials:
Since the concentration of trace elements affects citric acid production profoundly, various techniques have been used to minimize the concentration of these metals in fermentation media. Complete elimination of trace elements is practically impossible, especially when raw materials such as molasses are used.
In recent years therefore, two approaches have been used to overcome this difficulty – firstly, pretreatment of raw material with chemicals, ion exchange resins, etc., to reduce the trace element concentration; and secondly, the development of strains of fungi that have the ability to produce citric acid in the presence of a high concentration of trace elements.
The use of chemicals such as potassium ferrocyanide to reduce the concentration of Fe++ in fermentation medium was first described by Mazzadroli (1938). Since then, this method has been extensively used for the reduction of Fe++ concentration in raw materials such as molasses, etc.
There are two methods by which ferrocyanide can be used for reducing Fe++ concentration- (1) by addition of ferrocyanide directly into the fermentation media at a low, non-growth inhibitory concentration, or (2) by treatment of molasses media with a high concentration of ferrocyanide before inoculation.
Ferrocyanide at high concentrations is toxic to fungi, and a high level in the growth medium will precipitate Fe++ and Zn++ and cause a deficiency of these metals. The concentration of ferrocyanide required for molasses clarification depends on the type of molasses.
Kovats (1960) has reported the use of 0.04-0.6% of ferrocyanide at pH 2.2 while others have suggested a concentration of 0.005-0.020%. These concentrations cannot, however, be taken as a rule and will depend on the raw material, used.
Leopold and Valtr (1967) suggest the following pretreatment of molasses with ferrocyanide to reduce the trace metal content. To 8 kg of molasses in 6.5 liters of water, 150 g of potassium ferrocyanide is added and boiled for 30 min. To this, H2SO4 is added to adjust the pH to 6.5-7.2. Twenty-five ml of phosphoric acid (45%) is then added to the mixture and again boiled for 20 min. The mixture is cooled and diluted to adjust sugar concentration to 15%. A nitrogen source is added and the medium is used for citric acid fermentation.
In the alternate method, ferrocyanide is added to the fermentation medium at the time of inoculation. Hustede and Rudy (1976) added about 1.2 g of ferrocyanide per liter of molasses medium containing NH4H2PO4 adjusted to pH 5.0, before inoculation with A. niger spores. The yield of citric acid under these conditions was nearly 89%.
Clark (1962) has also reported that adding ferrocyanide to the fermentation medium at a concentration of 20-40 ppm was adequate. It is, however, suggested that during the time of high acid production, the concentration of ferrocyanide should not exceed more than 20 ppm.
The concentration of ferrocyanide tolerated by A. niger depends on the stage of fermentation; for example, during the initial growth period, a concentration of ferrocyanide as high as 10-200 mg/ml is tolerated, while during the acid production stage, the concentration must be below 20 μ g/ml.
The addition of potassium ferrocyanide does not affect the carbon, nitrogen, or phosphorus content of the substrate but only reduces the ash content. Metals known to interfere with citric acid production, mainly Fe+ + and Zn++, are precipitated. Whether citric acid production can be increased by adding ferrocyanide to the fermentation in parts before and after sterilization of the medium has also been examined. Leopold and Valtr (1969) have found that when two-thirds of ferrocyanide is added before and one- third after boiling, the yield of citric acid by A. niger is increased.
Other chemicals that are used for reducing the metal content of molasses are the chelating agents such as EDTA, activated charcoal, and polyethylene amine.
Use of quaternary ammonium compounds, such as diisobutylphenoxyethyl dimethylbenzylammoniumchloride, to a molasses medium has been reported to increase the yield of citric acid by 92%. Mints et al. (1976) have reported that addition of trilon B at 100-500 mg/liter to a molasses medium in addition to ferrocyanide improved the yield of citric acid by A. niger.
The use of ion exchange resins for reduction of the metal content of molasses media or sugar solutions has been commonly practiced in the production of citric acid, and it appears to be superior to the chemical treatment. However, clarification of complex materials such as molasses may pose certain problems.
pH:
Generally, a neutralizing agent such as CaCO3 is not used in citric acid production by fungi since these organisms can tolerate substantial amounts of acidity. The maintenance of a favorable pH is, however, very essential for the successful production of citric acid. The initial pH required depends on the carbon source used. For example- when sucrose or glucose or clarified molasses or other relatively pure materials are used, a low pH (3.0) is desirable.
A higher initial pH leads to the accumulation of oxalic acid. When crude molasses is used as a carbon source, a higher pH is desirable. In fact, a low pH in cane molasses medium has been found inhibitory for the growth of A. niger.
However, when decationized molasses is used, the initial pH can be 1.4-2.8. A low initial pH has the main advantage of preventing contamination, suppressing oxalic acid formation, and making the sterilization operation more efficient. Inorganic acids such as HCl or H2SO4, and NaOH are used to adjust the pH of the fermentation media.
A typical composition of basal media used for citric acid production by A. niger is as follows:
Inoculum Development:
Citric acid-producing fungal strains are generally maintained on standard media for fungi although stocks may be preserved in soil, silica gel, glycerol, etc. The inoculum for fermentation can be either the spores or the pre-grown mycelia. When spores are used as the inoculum, a suspension of spores is freshly prepared in sterile water or sterile water containing Tween 80 and this is used to inoculate the fermentation medium.
The initial concentration of spores may vary between 1 x 105 and 1 x 107/ml of fermentation media. For practical reasons, in surface and solid state culture fermentations, a spore inoculum is generally used. In surface culture fermentation, after the spores are mixed with the fermentation medium, the fungus grows on the surface of the liquid. This mycelial mat can be reused as in the “replacement culture technique.”
In submerged fermentations, spores as well as mycelial pellets are used as inoculum. When mycelial pellets are used, spores are first inoculated into a growth medium and allowed to grow under submerged conditions for 2-3 days. The mycelial pellets are then used as inoculum for larger fermentations.
When pre-grown mycelia are to be used as inoculum as in a large fermentation, the inoculum and fermentation media are of the same composition. In fact, the use of complex media such as wort or koji has been found to be inferior as media for inoculum development.
Spore viability varies with the age of the spores and, as a consequence, the germination properties also change with age. There have been very few studies made to assess the effect of the age of spores on fermentation abilities of A. niger. Chaudhary et al. (1978) have found that the spores from a 3-day-old slant culture are as good as spores from a 7-8-day-old slant culture for citric acid production.
This suggests that the age of the spores may not have a bearing on citric acid-producing ability. However, it is difficult to determine the exact age of a population of spores derived from slant cultures since a culture of A. niger sporulates in 24 – 36 hr. Whether spores formed very early are as effective as those formed late is not known. Also, 7-8 days is too short a period to assess the effect of spore development on efficiency.
The composition of the sporulation medium and the source of spores have been found to affect subsequent performance. For example, spores produced under submerged fermentation were found to be poor acid producers, while spores from a surface culture were found satisfactory.
Miles Lab. (1958) has patented a process for citric acid production in which the inoculum for submerged fermentation was prepared by inoculating moist sterile maize bran with A. niger spores. The mixture was incubated for 7 -14 days at 28°- 30°C and this when used as inoculum was found to be superior to the spores grown on other media.
Aeration, Temperature, and Time of Incubation:
The citric acid fermentation is essentially an aerobic fermentation and the organism needs an abundant supply of oxygen beyond that required for growth. The organism depends on the terminal oxidation of the reduced coenzymes of the respiratory chain by oxygen. Therefore, it is absolutely necessary to ensure an adequate supply of oxygen during the fermentation.
Aeration and agitation are primarily employed in liquid fermentations to ensure an appropriate oxygen supply as well as to maintain ventilation and prevent contamination by providing a positive pressure inside the fermenter. The degree of agitation and aeration will depend upon the organism, the medium, and the size of fermenter. It is generally agreed that the oxygen demand of a fermenting culture is so high that the amount of oxygen in a saturated aqueous medium is inadequate.
This has made it essential to maintain an appropriate supply of aeration and agitation in a fermenter during the fermentation. In recent years, this aspect of aeration in fermentation has received considerable attention, but there are problems yet to be solved both in the theory of oxygen transfer and in the application of this theory to tanks containing thousands of liters (gallons) of fermentation material.
Three ways in which aeration can be ensured are- (1) surface cultivation where oxygen diffuses through the surface which is maintained in a static condition; (2) by submerged aeration in which sterile air or oxygen is pumped from beneath the surface of the culture; and (3) shake culture, which is a process of both surface as well as submerged aeration. This process is, however, not feasible on an industrial scale.
Citric acid fermentation by surface culture is carried out in shallow pans of high grade aluminum or stainless steel containers. The volume of media is determined by the strain and other conditions. Under these conditions growth occurs on the surface of the medium and conversion of sugar to acid occurs intra-cellularly before it is excreted. Thus, the rate of the diffusion process will determine the time of citric acid accumulation.
Agitation of the medium by gentle or moderate shaking under surface culture conditions has been found to retard citric acid production in strains suitable for surface fermentation. However, the flow of a small amount of air over the fungal mats has no deleterious effect. Although in surface culture humidified air is blown over the trays, the amount of the air supply has to be predetermined.
Under submerged culture conditions, either oxygen or air is continuously supplied to the growing culture and a limitation in the supply of oxygen reduces citric acid production. As in surface culture conditions, the oxygen supply should continue at an optimal rate throughout the fermentation. Generally, strains of fungi suitable for surface fermentation do not perform equally well in submerged culture suggesting that oxygen requirements are different for different culture conditions.
This necessitates the development of cultures for different processes. Usami et al. (1960) have determined the effect of aeration on citric acid production by A. niger in 5 liter jar fermentors. With an increase in the aeration rate, the fermentation time was decreased and the yield of citric acid was increased. The effective aeration rate was found to be 3 x 10-6 g moles O2/ml/min or higher. Under these conditions the citric acid yield was 68%.
In submerged fermentors either purified compressed air or oxygen along with agitation is used. The cost of production may not be significantly affected when air is used, but when purified oxygen is used, the cost of production increases. In order to reduce the cost of production but at the same time retain the efficiency, the possibility of reusing fermentation gases has been examined.
Clark and Lentz (1971) re-circulated fermentation gases from a submerged fermentation of ferrocyanide-treated beet molasses using O2 for aeration after removal of carbon dioxide and found that this had no effect on citric acid production.
Aeration and agitation in submerged culture fermentation not only provide necessary oxygen for the growth of the organism but also promote efficient excretion of citric acid into .the fermentation medium. Wendel (1967) has shown by electrical conductivity measurements during citric acid production that in the initial stages of fermentation citric acid diffuses slowly into the medium.
The stratification that occurs on the mycelium during the period inhibits fungal metabolism and decreases the rate of citric acid production. By a combination of stirring and circulatory pumping applied to the fermentation medium, such stratification has been prevented, allowing metabolism and efficient citric acid production.
Interruption of aeration during fermentation under submerged conditions affects acid production; however, the extent of damage depends upon the duration of the interruption and the phase of fermentation. For example- a 30 min interruption of a 24-hr-old fermentation decreased the acidity of the medium by 13%, a 60 min interruption by 20% and a 7.5 hr interruption by 60%.
This suggests that the older the fermentation, the more sensitive it is to interruption of aeration. Apparently, in later stages when the oxygen supply becomes limiting, citric acid is re-metabolized by the organism. Our current knowledge of the actual site of dislocation of the fermentation during breakdown in aeration is not known. It is therefore safer and more economical to devise preventive measures rather than experimenting on recovery conditions for recovering dislocated fermentations.
In the solid state fermentation, the fungus is allowed to grow in a medium impregnated in a carrier. Under optimal conditions, because of the increased surface area exposed, this system provides an ideal supply of oxygen for the growth and citric acid production.
As stated earlier, aeration conditions and oxygen requirements for each culture under different sets of conditions have to be determined. The amount of oxygen required for efficient conversion has to be determined for the strain of fungus and the cultural conditions, based on the ratio of surface area to volume at which maximum conversion of sugar into citric acid occurs.
A. niger and other fungi used in citric acid fermentation have an optimum temperature between 25° and 30°C. An increase in the temperature of incubation beyond 30°C has been found to decrease the citric acid yield and increase the oxalic acid accumulation, irrespective of the fermentation conditions; therefore, maintenance of optimum temperature is necessary for optimum citric acid production.
The optimum time of incubation for maximal citric acid production varies both with the organism and the fermentation conditions. In the surface culture, the fermentation is usually complete in 7-10 days while under submerged conditions the incubation period is much shorter (4-5 days).
Attempts have been made to decrease the fermentation time under both surface and submerged culture by altering the cultural conditions. Meyrath and Ahmed (1966) have attempted to reduce the fermentation time by the addition of vermiculite and found that the time can be reduced from 10 to 5 days.
In submerged fermentation conditions with the current methodology, 4-5 days appears to be the minimum required. As in other fermentations the incubation period has to be adequate for allowing growth and product formation. Since the fungi are relatively slow-growing organisms, it is doubtful if incubation periods less than 3-4 days will be adequate.
Under solid state fermentation conditions, a period of 6-7 days is recommended. However, this process has not been rigorously tested on a large scale to arrive at precise conclusions.
Additives and Stimulants:
A variety of stimulants have been tested for improving citric acid yields by A. niger. The most important of these is methanol (CH3OH). The use of methanol in citric acid production was first reported by Moyer (1953). Since then it has been used in surface, submerged, and solid culture fermentations for increasing yields of citric acid by A. niger. There have been several reports which are consistent with Moyer’s findings, that among alcohols, methanol and, to a smaller extent, ethanol are beneficial in increasing citric acid yield.
The effect of methanol in increasing citric acid yield appears to be a general phenomenon in strains of A. niger. This chemical at a concentration of 3-4% has been found to retard growth, delay sporulation, and increase citric acid yields. For methanol to be beneficial, it is essential that this be added to the medium before inoculation. Addition of methanol after 24 hr or later has not been found to be beneficial.
This suggests that methanol perhaps has some role in conditioning the mycelia without impairing their metabolism. Earlier, it was suggested that methanol perhaps increases the tolerance of fungi to trace elements such as Fe, Mn, Zn, etc. The derivation of mutants that tolerate a high concentration of trace metals but still respond to methanol addition is inconsistent with this assumption. It is likely that methanol also affects the permeability properties and enables greater excretion of citric acid.
The effect of other chemicals with no nutritional value in improving citric acid production has also been examined. For example, inhibitors of metabolism such as CaF, NaF, KF at a concentration of 10-4 M have been found to accelerate citric acid production. Similarly, malic hydrazide (1 g/2.5 kg molasses) under submerged culture conditions has been found to increase citric acid production by A. niger.
In the absence of this chemical, the yield of citric acid was 30% while in its presence, it was 70%. Addition of mild oxidizing agents such as hydrogen peroxide or naphthoquinone or methylene blue to the fermentation medium has also been reported to stimulate acid production. The exact mechanism by which these affect citric acid production is not known.
Other chemical additives, such as aromatic amides, esters of dichloroacetic acid, sodium sulfite, and crysyllic acid, have also been tested and their effect appears to be more in controlling contamination from other microorganisms than in controlling citric acid fermentation.
Short chain carbohydrates such as glycerol or lipid materials or other metabolizable complex compounds have been examined for their effect on increasing citric acid yields by A. niger. Addition of glycerol to a molasses medium at a rate of 30-50 g/liter has been found to increase citric acid yield by nearly 30%. Similar results were also obtained when sorbitol, mannitol, or erythritol were added to the medium. Glycerol when added was found to be metabolized and utilized for citric acid production.
The use of lipid materials such as various vegetable oils, fatty acids, etc., has been reported to be beneficial for citric acid production. Millis et al. (1963) reported that addition of fatty acids of chain length of 15 carbon atoms or fewer or natural oils containing a high amount of unsaturated fatty acids such as corn oil, almond oil, linseed oil, peanut oil, etc., increased citric acid yield by 20% (Table 16.4).
It is suggested that unsaturated fatty acids may also serve as alternate hydrogen acceptors during citric acid fermentation in addition to serving as antifoam agents and stimulants. Similar results on the use of refined peanut oil in molasses medium or cane juice medium for improved citric acid yields have also been reported by Dhankar (1972) and Kumar and Ethiraj (1976).
Antifoam agents such as octadecanol (0.75% solution) or Antifoam AE (silicone oil) have been tested and found to be effective in controlling foaming and increasing the yields of citric acid. Apparently, the presence of antifoam agents allows increased aeration and agitation.
Complex components such as mycelial digests, bakers’ yeast, rice bran, etc., have been tested for their usefulness in increasing citric acid yields. A Belgian patent describes the use of a mycelial digest from A. niger for increasing citric acid yields. The stimulatory component from the digest has been isolated by chromatography on anion exchange resin or cellulose.
The yield of the stimulant is increased by the addition of sulfonamides or streptomycin. The stimulant is dialyzable and thermostable, but unstable in alkali. Addition of this stimulant to a sucrose medium increases citric acid production by 60-70% while without the stimulant the yield is 40-50%. Suprisingly, the stimulant can be prepared from A. terreus and A. niger as well as from Escherichia coli.
Bakers’ yeast when added to the fermentation medium has been found to improve the yield of citric acid by A. niger. Leopold (1965) has patented a process in which the addition of 1.92 mg of pressed bakers’ yeast to a culture medium and cultivation for 9 days at 32°C increased the yield of citric acid from 61.5 to 72.1%.
Cyclic AMP, which has been of interest in recent years, has also drawn the attention of investigators in citric acid production. Wold and Suzuki (1973) have reported that a strain of A. niger accumulated citric acid in the medium when cAMP concentration was 10-6 M or higher (Table 16.5). The rate of citrate synthesis under these conditions was found to be increased.
Adenosine, ATP, and/or cGMP also stimulated citric acid production at a concentration of 10-3 M, but were ineffective at 10-4 M. AMP had no effect while GMP and guanosine inhibited citric acid production slightly. ADP on the other hand strongly inhibited citric acid accumulation. Addition of thiophylline along with cAMP was found to increase the effect of cAMP. It is suggested that citric acid production in fungi perhaps results from an abnormal cAMP metabolism.
Normally the fermentation medium is sterilized prior to inoculation. When sucrose or deionized molasses is used as the carbon source, the pH of the medium is also kept low and this is adequate to prevent bacterial contamination. However, when crude molasses media are used, necessitating a higher pH, the chances of bacterial contamination are greater.
To prevent this, antiseptics such as pentachlorophenolate, formic aicid, 5-nitro- 3-furaldehyde semicarbazone, tetracyclines, etc., have been used. It is claimed that these chemicals effectively control bacterial growth without affecting the citric acid producing ability of A. niger.
Citric Acid Production by Yeasts:
Until recently, almost all the citric acid produced by fermentation was manufactured using A. niger and a few other fungi. It is now well known that many kinds of yeasts also accumulate citric acid in their growth media along with relatively large amounts of isocitric acid.
The variety of yeasts known to produce citric acid from various sources are species of Candida, Hansenula, Pichia, Debaryomyces, Torulopsis, Kloeckera, Trichosporon, Torula, Rhodotorula, Sporobolomyces, Endomyces, Nocardia, Nemato- spora, Saccharomyces, and Zygosaccharomyces. Out of these yeast genera, the species of Candida (Saccharomycopsis) are the ones that are widely used for studies on citric acid production.
These species include C. lipolytica, C. tropicalis, C. zeylanoides, C. fibrae, C. intermedia, C. parapsilosis, C. petrophilum, C. subtropicalis, C. oleophila, C. hitachinica, C. citrica, C. guilliermondii, and C. sucrosa. A variety of substrates have been used for producing citric acid by yeast. These include glucose, acetate, hydrocarbons, molasses, alcohols, fatty acids, and natural oils.
Citric acid production from glucose is carried out in media containing glucose (10-18%), NH4Cl, KH2PO4, and MgSO4.7H2O, and a neutralizing agent such as CaCO3. The concentration of various nutrients in the medium depends on the yeast strain used. Fermentation is always carried out under aerobic conditions, and a temperature between 22° and 30°C is optimum.
During fermentation, isocitric acid is accumulated along with citric acid and the amount of isocitric acid produced depends on the cultural conditions and the strain of yeast. Generally, yeast strains producing the least amount of isocitric acid but high levels of citric acid are desirable. The time of fermentation varies from 3 to 6 days.
The metabolic pattern of these yeasts makes them ideal for use in citric acid production. These yeasts are highly oxidative and therefore need a considerable amount of aeration for their growth and metabolism. Fermentations using these yeasts are therefore carried out with vigorous agitation.
Oh et al. (1973) have reported citric acid production by a strain of Hansenula anomala in a 10% glucose medium containing 3% CaCO3 at a temperature of 30°C with agitation at 110 rpm. Potassium dihydrogen phosphate (0.05%) and magnesium sulfate (0.025%) are also added. Ammonium chloride at 0.1% concentration was most effective as a N source. In 6 day fermentation, 46% citric acid was produced.
Ishi et al. (1972) have developed a process for the production of citric acid by fermentation of waste glucose (32.6% glucose, 3% fructose) left after separation of fructose from a fructose-glucose mixture, after the addition of urea, corn steep liquor, KH2PO4, MgSO4, MnSO4, and CaCO3. The medium was inoculated with C. oleophila and fermentation was carried out at 28°C under aeration for 5 days to yield 70.5% calcium citrate based on the initial carbon content.
Citric acid production from molasses by species of Candida has also been reported. Using C. guilliermondii and C. lipolytica, a continuous process for citric acid production from molasses has been described. The medium contains 50-250 g/liter sugarcane or beet molasses; a nitrogen source such as (NH4)2SO4, NH4Cl, or NH4NO3; minerals; and vitamin B complex.
Fermentation is carried out for 189 hr with 0.3-1.5 vol. air/min. Initial pH of the medium is 5.5-6.5 which is stabilized at 2.8-4.0 during citrate formation. From 3145 kg sugar, 1119 kg of citrate monohydrate was produced. Similarly, Liu (1975) has also reported citric acid production using Candida spp. in a medium containing molasses with yields as high as 50%.
Various alcohols such as methanol, butanol, ethanol, and C12-16 alcohols can serve as carbon sources for citric acid production by strains of C. fibrae, C. subtropicalis, Pichia farinosa, C. lipolytica, and Torulopsis xylinus.
The initial concentration of alcohol in the medium varies from 1 to 2% and additional alcohol is added during the fermentation to maintain the concentration. Besides alcohol, the medium should contain a nitrogen source, a phosphate source, and Fe++, Mg++, Zn++, Mn++, and Cu++ as trace elements. Fermentation is carried out for 7 days and yields of 27.9 g citric acid/liter are obtained.
Production of citric acid from acetate by yeast has been described by various workers. Species of Candida such as C. zeylanoides, C. parapsilosis, C. fibrae and C. subtropicalis are cultivated using acetic acid or calcium acetate as a carbon source. Fermentation is carried out for about 3 days at a pH of 5-6 with shaking.
Fatty acids, natural oils, and fats have been tested for citric acid production utilizing various genera of yeasts like Candida, Hansenula, and Pichia. Tallow, coconut oil, palm oil, olive oil, soybean oil, linseed oil, rape seed oil, fish oil, corn oil, and free fatty acids have been tested for this purpose.
A typical medium for citric acid production from coconut oil is given below:
Fermentation is carried out at 30°C with shaking for 84 hr to give a 146% yield of citric acid from palm oil with a mutant strain of C. lipolytica. Various strains of C. lipolytica have been extensively investigated for citric acid production from hydrocarbons such as n-paraffins, n-alkanes, and alkenes.
Strains of C. lipolytica yielding a high amount of citric acid without the formation of isocitric acid have been developed by mutagenic treatment.
Several processes for citric acid production from n-paraffins have been described. The process involves cultivation of mutants of Candida with high citric acid-producing ability in an n-paraffin-containing medium containing nitrogen, phosphate, MgSO4, CaCO3, and vitamins. Fermentation is carried out with shaking at 28°-30°C for 4-6 days. A typical medium for citric acid production from n-paraffins includes hydrocarbons, 40-60 g; NH4Cl, 2 g; KH2PO4, 0.5 g; MgSO4, 0.5 g; corn-steep liquor, 1 g; and CaCO3, 30 g/liter.
As with A. niger fermentations, the concentration of ferric ion in the medium affects the accumulation of citric and isocitric acids. An increase in citric and a decrease in isocitric acid can be achieved by adjusting the iron content to very low concentrations. Since the maintenance of a low concentration of iron in complex media is difficult, fluoroacetate-sensitive strains of C. lipolytica with low aconitase activity have been developed which produce increased amounts of citric acid from n-paraffins.
Small amounts of polyhydric alcohols such as mannitol, sorbitol, or erythritol are detected temporarily at early stages of the fermentation in an n-paraffin culture medium containing a neutralizing agent such as CaCO3. In a culture medium containing no neutralizing agent, the amount of citrates is decreased.
The concentration of thiamin in the fermentation medium has a marked effect on the amount of citric and isocitric acid produced. With sufficient thiamin in the medium a large amount of citric acid is produced, while in a thiamin restricted medium a large amount of α-ketoglutarate is produced with a reduced amount of citrate.
Citric acid production from n-alkanes is similar to that from n-paraffins. A typical medium for citric acid production from alkanes using strains of C. lipolytica includes hydrocarbon, 4-6%; NH4Cl, 0.2%; KH2PO4, 0.05%; MgSO4.7H2O, 0.05%; corn-steep liquor, 0.1%; and CaCO3, 3%. The fermentation time was 6-8 days under shake culture conditions and the yield of citric acid was 17-56%, depending upon the alkane.
A unique medium containing a lead salt has been described for citric acid production by yeast. In a French patent, citric acid production by Candida in aqueous nutrient media containing 0.5-1.5 g lead oxide or lead salts has been described. A medium containing cerelose, 150 g; CaCO3, 10 g; NaCl, 4 g; yeast extract, 5 g; peptone, 15 g; CaCO3, 10 g; and lead acetate, 1.5 g/liter was seeded with an inoculum of C. guilliermondii and incubated for 72 hr at 30°C under aeration. It yielded 53 g citric acid/liter. Without lead acetate the yield was only 29 g. The role of the lead acetate in this fermentation is not clear.
One advantage of using yeasts for citric acid production, as compared to aspergilli, is the tremendous potential for developing a continuous process. In fact, a continuous process for citric acid production using Candida species has been patented by Miall and Parker (1975).
Citric Acid Production by Bacteria:
Despite the availability of a large amount of information regarding the biochemical activities of bacteria, this group of organisms has not been vigorously exploited for the production of citric acid. In the past 10 years only about 10 references have appeared and most of these are patents. Hence, few details are available regarding this aspect of citric acid production.
Some bacteria such as Bacillus licheniformis, Bacillus subtilis, and Brevibacterium flavum have been found to possess the ability to produce citric acid from either glucose, isocitric acid, or from hydrocarbons. Sardinas (1972) has patented a process for producing citric acid using B. licheniformis from glucose-containing media. Fermentation is carried out under aerobic conditions at 30°-37°C for 36-120 hr at a pH of 7.0. Besides glucose, the medium is supplemented with a nitrogen source such as urea, ammonium sulfate, or glutamate in addition to salts and calcium carbonate.
Yields up to 42 g/liter have been reported. Kyowa Fermentation Industries have patented a process for the production of citric acid by Arthrobacter paraffinens. The organism is cultured in an aqueous medium containing dodecane or a mixture of C12-C14 paraffins in addition to salts after pre-culturing in an inoculum medium. The fermentation medium was inoculated at a 5% level and aerated with 3 v/v/min at 28°C for 72 hr to yield 28 mg citric acid/ml.
Using n-paraffins as a carbon source, citric acid is also produced by Corynebacterium sp. and Brevibacterium sp. Fukuda et al. (1970) have patented a process for citric acid production which involves culturing various species of Corynebacterium in paraffin-containing media. Yields of 41.4 mg of citric acid/ml have been obtained by culturing for 64 hr at 32°C.
Alternate methods of producing citric acid by growing bacteria such as Klebsiella, Aerobacter, Pseudomonas, Micrococcus, Bacillus, Brevibacterium, Corynebacterium, and Arthrobacter in media containing isocitric acid have also been reported. The organisms are cultured in media containing 5-6% isocitric acid at a pH of 5-8 for about 2 days at 32°-37°C under shake culture conditions to convert isocitric acid into citric acid.
A mutant of Brevibacterium flavum requiring L-glutamate for growth has been described to produce citric acid when cultured in a medium containing glucose, 3.6%; urea. 0.2%; KH2PO4, 0.01%; MgSO4, 0.04%, sodium L-glutamate, 0.05%, and CaCO3, 1%, in addition to soybean hydrolysate (3 ml/ liter) and minor amounts of Vitamin B12, FeSO4, and MnSO4. After incubation at 30°C for 4 days at pH 7.4, the yield of citric acid was 50 mg/ml.
These studies on bacteria, although few in number compared to the reports on fungi, have opened a new avenue for citric acid production.
Genetic Improvement in Citric Acid-Producing Microorganisms:
Despite great advances in our knowledge of the genetics and biochemistry of microorganisms, the application of genetics to the improvement of microorganisms producing citric acid has not been very significant. The main technique used for genetic improvement of citric acid-producing fungi has been the age-old technique of mutation and selection. Normally, a spore population is suspended in a liquid medium or a buffer and subjected to mutagenesis, and the survivors are screened for their ability to produce citric acid.
One difficulty in screening mutants for better citric acid producers is the lack of a precise and quick method by which citric acid-producing strains can be easily selected. Agar plates with nutrient media containing an indicator have been used for preliminary screening of survivors for citric acid-producing mutants.
This method is, however, not very precise. It is well known that the type of acid produced by fungi is pH dependent and when agar medium of pH 6-7 containing sucrose is used for detecting acid producers, normally oxalic acid-producing mutants are detected.
To obtain mutants that produce more citric acid in molasses medium, agar medium containing smaller concentrations of molasses and an indicator have been used. By this method it has been possible to isolate mutants producing more citric acid in molasses medium.
Citric acid production is extremely sensitive to the concentration of trace elements, especially iron. Citric acid production in a molasses medium is more economical than in a sucrose medium. However, the control of the concentration of trace elements in such complex media is difficult and therefore attempts have been made to isolate mutants that would tolerate a high concentration of trace elements and produce large quantities of citric acid when cultured in crude cane molasses medium.
Mutants of A. niger that have better ability to produce citric acid have” been derived by mutagenesis and extensive screening. Trumphy and Millis (1963) reported the isolation of one mutant of A. niger, after screening nearly 40,000 survivors, which produced 4 times more citric acid than the parent culture. Shcherbakova (1964) has reported the isolation of A. niger mutants by UV irradiation.
These mutants produce equal amounts of citric acid in synthetic media, but in a medium containing molasses, they produce 1.5 times more citric acid than the parent. Das and Nandi (1969) have also reported the isolation of mutants of A. niger using UV, gamma-rays, and nitrogen mustard. The most promising mutant was the gamma-ray mutant which produced about twice the amount of citric acid as the parent culture.
This mutant was further subjected to UV irradiation and a second stage mutant was produced which gave 3 times more citric acid than the parent culture. Kahlon and Vyas (1971) have reported the screening of 800 survivors of nitrous acid and UV mutagenesis.
Of these, one mutant produced a large amount of citric acid in cane sugar medium while another produced good amounts of citric acid only in molasses medium. Banik (1975) has reported the derivation of mutants of A. niger by sequential mutagenesis using UV and ethyleneamine, which had the ability to produce about 70.5 mg of citric acid/ml in a sucrose medium while the wild type produced only 11.6 mg/ml.
Similarly, Sharma (1973) has reported the isolation of a mutant of A. niger by sequential mutagenesis of A. niger spores using nitrous acid, nitrosoguanidine, and UV light. This mutant has been found to produce more citric acid than the parent when cultured in a molasses medium.
Besides induction of mutants using various mutagens, strain improvement in A. niger by somatic recombination has been attempted, but without much success.
Yeasts, particularly the members of the genus Candida, are increasingly being used for citric acid production because of their ability to produce citric acid from a variety of substrates such as glucose, molasses, hydrocarbons, acetate, alcohols, lipids, etc. In these fermentations citric acid is produced along with isocitric acid. The development of mutants that accumulate only citric acid in high concentration without concomitant accumulation of isocitric acid has been attempted.
Also, mutants with either low aconitase activity or those with an enzyme sensitive to fluoroacetate have been isolated and used for citric acid production. Ikeno et al. (1975) have studied various Candida mutants derived by nitrosoguanidine mutagenesis for citric acid production in glucose, n-paraffin, or coconut oil-containing media (Table 16.7).
These mutants produce higher amounts of citric acid in glucose, n-paraffin, or in coconut oil-containing media as compared to the parent culture. Sriprakash (1977) has isolated a mutant of C. lipolytica which accumulates 140 g of citric acid/liter, representing a conversion of about 70% on the basis of the carbohydrate input. The ease with which yeasts can be handled and the high yields reported make them ideal test systems for genetic improvement studies.