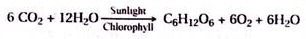ADVERTISEMENTS:
The following points highlight the top eleven experiments on photosynthesis in plants. Some of the experiments are: 1. Simple Demonstration of Photosynthesis 2. To Study the ”Primary Photochemical Reaction” of Photosynthesis 3. To Study the “Dark Reaction” of Photosynthesis 4. To Study the Essentiality of the Factors for the Photosynthetic Process and Others.
Experiment # 1
Simple Demonstration of Photosynthesis:
(a) With the help of a beaker and a funnel:
ADVERTISEMENTS:
Experiment:
A large beaker of capacity 500 ml is taken and are filled two-thirds with distilled water containing 0.1 % KHCO3 which acts as a source of CO2. Some fresh and healthy aquatic plants like Hydrilla are taken in a beaker and the plants are cut obliquely at their bases under water.
Cut ends are tied together with the help of a thread and are kept towards the neck of an inverted funnel in such a way that the limb of the funnel almost covers the Hydrilla plants and the stem of the funnel remains about one centimeter under the water surface.
The whole set-up is now exposed to bright light and observed from time to time. Another set-up is similarly prepared and kept under a very low light intensity.
ADVERTISEMENTS:
Observation:
It is observed that evolution of bubbles from cut ends of the plants takes place in the set-up exposed to light. Little evolution of bubbles takes place in the set-up maintained in low light intensity.
Inference:
In light, evolution of oxygen bubbles takes place due to photosynthesis. This is further proved by the fact that little evolution of bubbles takes place in the set-up placed in low light intensity.
(b) With the help of Wilmott’s bubbler:
Experiment:
The apparatus consists of a flask of capacity 500 ml fitted with a rubber cork having a central hole through which passes a glass tube. The lower end of the tube reaches the middle of the flask while its upper end forms a jet within a cylindrical cup. A graduated tube having a stopcock at one end remains inverted over the jet (Figure 27).
Now cut ends of one or two Hydrilla plants are introduced within the lower end of the glass tube. The flask is filled with distilled water and a small amount of bicarbonate is added to it. The Hydrilla plants with the tube are immersed within the flask and the rubber cork is fitted tightly.
ADVERTISEMENTS:
The upper cylindrical cup is filled with water so that the jet remains well below the water level. The graduated tube filled with water is inverted over the mouth of the jet and clamped properly. Two such set-ups are arranged and one is kept in bright light and the other in low light.
Observation:
Same as in Expt. 1(a).
Inference:
ADVERTISEMENTS:
Same as in Expt. 1(a).
N.B. The size of the bubbles can be adjusted conveniently by varying the oblique surface of the cut ends of the Hydrilla plants.
Experiment # 2
To Study the ”Primary Photochemical Reaction” of Photosynthesis:
(a) To study photolysis of water from evolution of oxygen:
ADVERTISEMENTS:
(i) Physical proof of oxygen evolution:
Experiment:
Two experimental set-ups are arranged as in Expt. 1(a). Now a test tube filled with water is inverted over the upper end of the stem of the funnel in each case, using the thumb to close the mouth of the tube while inverting.
Both the set-ups are placed in bright sunlight. As photosynthesis occurs oxygen bubbles are given out, which rise up in water through the funnel stem into the inverted test tube and collect at its top by displacement of water. When the gas has collected to a considerable amount it is tested for oxygen.
ADVERTISEMENTS:
One of the gas-filled test tubes (other is kept aside for the next experiment) is removed by using thumb to cover its mouth and the thumb is then removed over a burning splinter or match stick.
Observation:
The burning splinter or the match stick glows vigorously.
Inference:
Since the burning splinter or match stick glows in the collected gas, it evidently proves that the collected gas is oxygen.
(ii) Chemical proof of oxygen evolution by alkaline pyrogallate (qualitative):
ADVERTISEMENTS:
Experiment:
The gas-filled test tube from Expt. 2(a) is taken and placed in a petridish containing potassium pyrogallate solution.
Observation:
It is observed that the level of water gradually rises up in the test tube.
Inference:
Alkaline pyrogallate is a strong absorbent of oxygen gas. This absorbs the oxygen in the test tube which creates a vacuum, consequently water rises upward. Hence it proves that the evolved gas collected in the test tube during photosynthesis is oxygen.
ADVERTISEMENTS:
N.B. If the test tube is half-filled with gas, water in the test tube must be carefully excluded from the tube and the mouth of the test tube closed with the thumb is then placed on alkaline pyrogallate solution.
(iii) Chemical proof of oxygen evolution by indigo-carmine solution (qualitative):
Experiment:
A very dilute solution of the dye indigo-carmine is prepared with previously boiled and cooled distilled water.Now minute quantity of sodium or potassium hydrosulphite (Na2S2O4, 2H2O or K2S2O4, 2H2O) is very carefully added to the solution until the blue colour just disappears. (A dilute sodium hydrosulphite solution may be added drop-wise from a burette and addition of the solution is stopped as soon as the colour just disappears.
This will avoid the possibility of excess addition of the reductant). Two test tubes are completely filled with this reduced decolourised dye solution. A few fresh and healthy Hydrilla plants are put in each and corked carefully to exclude all contact with atmospheric air. One lube is exposed to bright light while the other is kept in dark.
Observation:
The solution of the first test tube which was kept in light soon turns blue whereas the second one which was kept in dark remains colourless.
Inference:
Indigo-carmine is auto-oxidisable, i.e., whenever reduced indigo-carmine comes in contact with air or oxygen it becomes oxidised. Indigo-carmine which is reduced to a colourless compound by sodium hydrosulphite is oxidised back with molecular oxygen to its original state having blue colour in case of the first test tube where evolution of O2 takes place due to photosynthesis.
In case of the second test tube where to photosynthesis takes place, it remains as such.
(iv) Chemical proof of oxygen evolution by Winkler’s test (quantitative):
Experiment:
Three burettes are taken. One is filled with 40% manganous chloride solution; second one is filled with KI + KOH (dissolve 175gm KOH and 37-5gm KI in 250 ml H2O) solution and the third one with conc. HCL.
Two Erlenmeyer’s flasks (250 ml) are filled with 150 ml previously boiled and cooled (to free from oxygen) water. To each flask 5 nil of 1-35% KHCO3 is taken.
A few fresh Hydrilla plants or green alga like Spirogyra, Chlorella or Scenedesmus are put to one flask only and both are kept in bright light. After one hour 5 ml of water from each flask is taken separately in two 100 ml flasks.
To each flask the following reagents arc added one by one:
A. 0.5 ml of 40% manganous chloride.
B. 1.0 ml of (KI + KOH) solution.
(It is stoppered quickly and carefully mixed by rotating the flask.)
C. 2 ml of conc. HCL.
(The precipitate of manganous hydroxide is re-dissolved.)
The dissolved oxygen now liberates free iodine from the KI present. It is then titrated against 0.01 N sodium thiosulphate in both the cases using 0.5 ml of 0.5 % starch solution till the blue colour disappears. Oxygen concentration in both the cases is calculated. During titration starch indicator should be used when a straw colour of the solution appears on titration with Na2S2O3.
Results:
The oxygen concentration is calculated from the following relation 1 ml of 0.01 N sodium thiosulphate (Na2S2O2, 2H2O) = 0.001 mg of oxygen or 0.0558 ml of oxygen. The initial oxygen concentration of water is calculated in both the flasks.
The final concentration is again calculated in each case. Difference between the initial and final values gives the increase in oxygen concentration in the medium. The result may be expressed as milligram of oxygen evolved per gram weight of the plant (fresh or dry) or as millilitre of oxygen evolved per hour by constant volume of the plant.
Discussion:
During this experiment a number of chemical reactions take place ultimately liberating iodine which is stoichiometrically equivalent to oxygen.
In the last step, the dissolved oxygen liberates chlorine which again liberates free iodine from KI present in the reaction mixture. The liberated iodine is then titrated against a standard sodium thiosulphate solution. This stands as an index of the oxygen content of the medium.
(i) Mn(OH)2 + 4HCl + O → Mn Cl2+ 3H2O + Cl2
(ii) 2KI + Cl2 → 2 KCl + I2
(iii) I2+ 2 Na2 S2O3 → Na2 S4O6 + 2 Nal.
N.B. If the concentration of Na2S2O3 used be 0.025(N) and a 200 ml aliquot of the sample is titrated, then the relation is: millilitre of Na2S2O3 E ppm dissolved oxygen.
(b) To study photolysis of water by demonstration of Hill reaction:
Experiment:
About 25 gm of spinach or lettuce leaves are homogenised in 50 ml of ice-cold 0.5 M sucrose solution. It is then filtered through double-layered muslin cloth or glass wool. The suspension is centrifuged at, low speed (500 rpm) for 10 minutes. The supernatant is re-centrifuged at 4000 rpm for 10 minutes. The sediment is taken and resuspended in 0.5 M ice-cold sucrose solution. It is then kept in dark.
The following solutions are taken in three test tubes:
All the test tubes are shaken and percentage of absorption is read in a colorimeter at 430 nm.
Tube 1 and tube 2 are then placed in light and tube 3 in dark. The percentage of absorption in each test tube is again measured at 15 minutes interval for 60 minutes. Before taking each reading, the test tubes should be shaken well.
Results:
The change in percentage absorption is noted in each case. The percentage of absorption decreases in case of tube number 2 due to reduction of dichlorophenol (reduced dichlorophenol is colourless). Whereas no change occurs in case of tubes 1 and 3.
Discussion:
The production of oxygen without accompanying CO2 fixation by isolated chloroplast, illuminated in the presence of substances like dichlorophenol-indophenol, ferric oxalate, ferricyanide, etc., the so-called Hill oxidants, is known as the Hill reaction.
The light energy absorbed by chlorophyll and accessory pigments is utilised in shifting up of water into hydrogen and oxygen.
The oxygen is liberated as gas while hydrogen is absorbed by Hill oxidant which gets reduced. This is photolysis of water (photolysis differs from electrolysis in that hydrogen gas does not appear simultaneously with oxygen gas but is used to reduce some compounds present in the chloroplast).
The present experiment shows that Hill reaction takes place in test tube number 2 where all the requirements of this reaction are fulfilled whereas in case of tube numbers 1 and 3, Hill reaction does not occur due to killing of chloroplast and absence of light respectively.
N.B. To prepare 6 5 pH buffer, 0.1 M disodium hydrogen phosphate and 0.1 M monosodium hydrogen phosphate are mixed in the proportion of 4: 6.
Experiment # 3
To Study the “Dark Reaction” of Photosynthesis:
(a) Evidence from study of temperature coefficients (Q10) at high and low light intensities:
Experiment:
An experiment is set up following the procedure of Expt. 1 (a) or 1 (b). If Expt. 1 (a) is followed, a glass jet having a slight bent at the tip is to be fitted to the short stem of the funnel by rubber tubing (proper care must be taken in drawing out the jet end from a glass tube, for, if the bending is not correct or if the jet opening is too small or too big, the evolved gas may accumulate there and does not come out at all.
For convenience a dropper may be used as a jet). A pinch of bicarbonate is added to the water of the beaker and the set-up is kept in bright sunlight. The temperature of water (say, X°C.) is noted by a thermometer.
Now the number of oxygen bubbles evolved and coming out through the fine jet-end is counted per unit time preferably a minute (it is advisable to discard very small bubbles coming out in torrents, as it is almost impossible to count them and better to select only relatively bigger bubbles of more or less uniform size).
Five such readings are taken and the average number of bubbles per minute at that particular temperature and light intensity is recorded. The temperature of water of the beaker is lowered to 10°C below that of first temperature, i.e., (X – 10) °C using ice blocks. The bubbles are similarly counted at that temperature and light intensity and average number of bubbles per minute is recorded.
The temperature of water is now raised to 10°C above that of the first temperature, i.e., (X + 10) °C using hot water or hot water bath. The bubbles are counted as usual at that temperature and light intensity and the average number per minute is recorded.
The same experiment is repeated at low light intensity, i.e., not under direct sunlight (if artificial light is used, the source may be removed to a distance to lower the light intensity). Bubbles are counted at each temperature at low light intensity and average number of bubbles per minute is recorded separately.
Results:
If the number of bubbles per minute at light intensity and at temperature of (X + 10) °C is a, the number of bubbles at X°C is b, and that at (X – 10) °C is c, then Q10 can be calculated as a/b and b/c. Average of these two gives the temperature coefficient of photosynthesis at that light intensity. Q10 is similarly calculated at low light intensity.
Discussion:
The temperature coefficient or Q10 of a chemical reaction or of a physiological process is the ratio of the rate of the reaction or process at a given temperature to the rate at a temperature 10°C below it. For purely photochemical reaction Q10 is about unity and for ordinary chemical reaction Q10 is at least two.
When photosynthesis is proceeding rapidly in well illuminated leaves receiving an ample supply of CO2, Q10 is ways greater than two. When the light intensity is low Q10 value is much less than two and sometimes approaching unity, i.e., values characteristics of photochemical reactions are obtained.
Thus in addition to at least one photochemical stage in photosynthesis there must be at least one chemical stage (dark reaction).
When the light intensity is high the temperature-dependent dark stage is limiting; when the light intensity is low, the photochemical stage is limiting and the whole process becomes relatively independent of temperature.
N.B. As majority of biological activities are catalysed by enzymes the Q10 law (Van’t Hoff’s law) is applicable to these bioprocesses within the temperature range of 0°C to 30°C.
Above SO2C reaction rate declines characteristically and the Q10 values decrease from two to nearly one. Thus Van’t Hoff’s law is not applicable at higher temperature, i.e., above 30”C as enzyme inactivation takes place more rapidly at higher temperature.
At higher temperature, i.e., above 30°C., the Q10 values must be carefully interpreted with particular attention to the effect of time. Because the rate of reaction may be doubled between 20°C. to 30°C. For a period of two hours, but if continued for several hours, the rate will decrease.
(b) Evidence from experiments in Intermittent Light:
Experiment:
Two experiments are set up as in Expt. 2(A). Initially both the set-ups are illuminated at the same light intensity and the rate of evolution of bubbles per minute is recorded. One is then kept in dark for 15 minutes with a bell jar covered with black paper.
After 15 minutes the bell jar is taken off and the rates of evolution of bubbles in both the sets are measured for 5 minutes. The same set-up which was in dark is again kept in dark for another 15 minutes. The bell jar is again taken off and rates are measured in each set. The experiment is repeated and four or five such readings are taken.
Results:
The average rate in continuous light and in intermittent light is calculated and results are represented in bar graphs.
Discussion:
The amount of photosynthesis per unit time (continuous rate) shown by green cells exposed to continuous illumination is less than the amount of photosynthesis per unit time of illumination (intermittent rate) by green cells exposed to alternating periods of light and dark.
The rate in intermittent light is increased as the light periods are shortened and sometimes become nearly double the rate in continuous light.
Evidently a given quantity of light energy is used in photosynthesis to a greater extent when supplied, not continuously, but in succession of small amount during short light periods. This happens because an essential phase of photosynthesis (dark reaction) goes on independently of the presence of light.
In intermittent light, this phase then proceeds in the dark periods as well as in light periods and consequently, the rate of photosynthesis per unit time of illumination is enhanced. Moreover, in continues light the photosynthetic product may inhibit the rate whereas in intermittent light the product may be used up in the dark phase.
N.B. In case of terrestrial plants the rate of photosynthesis is measured by weight-area method.
Experiment # 4
To Study the Essentiality of the Factors for the Photosynthetic Process:
(a) Light dependence of photosynthesis:
(i) By noting evolution of oxygen in light and dark:
Experiment:
Experiment is set up as in Expt. 1 (a) or 1 (b). It is kept in light for some time and then placed in dark.
Observation:
Only in light evolution of oxygen bubbles takes place. In dark no evolution of oxygen bubble is observed.
Inference:
Hence light is essential for photosynthesis, since without light no photolysis of water takes place and oxygen does not evolve.
(ii) By using Ganono’s light screen:
Experiment:
Leaves of a suitable potted plant are made starch-free by keeping the plant in dark for 24 to 48 hours. A starch-free leaf from this plant is then covered with Ganong’s light screen (Figure 28).
The main advantage of this light screen is that a part of the leaf is cut off from the light without hampering its ventilation. The whole plant with the light screen attached to one of its leaves is then kept in bright light.
After 24 hours the screen is taken off and the leaf is severed from the plant. It is then bleached with boiling water and then with 95 % boiling alcohol. A few drops of lactic acid may be added for better bleaching. After its complete decolourisation, the yellowed leaf is washed with 1 % iodine solution for about a minute or so.
Observation:
The exposed part turns blue whereas the covered portion (where light could not penetrate) remains yellowish red.
Inference:
The blue colour indicates the formation of starch a product of photosynthesis. Yellowish portion shows absence of starch. Hence the experiment proves the necessity of light in photosynthesis.
N.B. Instead of a light screen a suitable photographic negative, which must be bright with clear-cut differentiation into black and white areas, can be used. After fixing the negative with sufficient care on a suitable starch free leaf and exposing the leaf with the negative in strong light for a day or two and subsequently treating the decolourised leaf with iodine solution, reasonably good positive print could be obtained.
(b) CO2 dependence of photosynthesis:
(i) By using CO2 free water (in case of aquatic plants):
Experiment:
An experiment is set-up as in Expt. 1(a) using previously boiled and cooled distilled water (in order to free it from dissolved CO2).
It is then kept in bright light and observation is made regarding evolution of O2 bubbles. Now a pinch of KHCO3 is added to the distilled water and allowed to dissolve in it completely. Observation is again made regarding evolution of O2bubbles.
Observation:
No oxygen bubble comes out in CO2free distilled water but a number of oxygen bubbles come out when a pinch of KHCO3 is added.
Inference:
The aquatic plants with their inherent adaptability can effectively utilise dissolved CO2 in water for photosynthesis because they have no contact with atmospheric air. As the boiled distilled water is devoid of dissolved CO2, the Hydrilla plants cannot photosynthesise. But when this water is enriched with dissolved CO2 by adding bicarbonate, these plants can easily photosynthesise and as a result O2 bubbles come out.
(ii) By Moll’s experiment (in case of terrestrial plants):
Experiment:
A wide-mouthed bottle fitted with a split cork is taken. A small quantity of 20% KOH solution is poured in the bottle and clamped horizontally or placed on a stand. A suitable starch-free leaf from a healthy; potted plant is introduced through the split cork in such a way that half of the leaf remains inside the bottle and the other half outside it.
Care should be taken so that the leaf inside the bottle does not come in contact ‘ with KOH solution. All connections are made air-tight with Vaseline. The whole set-up is placed in bright sunlight (Figure 29).
After 2 to 3 hours the leaf is detached from the plant and carefully taken out from the bottle. The leaf is then washed with water, bleached perfectly and treated with 1 % iodine solution.
Observation:
The portion of the leaf remaining outside the bottle is coloured blue with iodine while the portion remaining inside the bottle takes no blue colour with iodine.
Inference:
The portion of the leaf remaining outside the bottle receives all conditions for photosynthesis (viz., CO2, light and chlorophyll) and so starch formation takes place normally which is evident from the blue colour with iodine.
The other half of the leaf which remains inside the bottle and where no CO2 is available due to absorption by KOH solution remains yellow showing no starch formation. This proves that photosynthesis cannot take place in an atmosphere completely devoid of CO2.
(c) Chlorophyll Dependence of Photosynthesis:
Experiment:
A plant having variegated leaves (croton, Coleus, etc.) is selected. Variegated leaves have green chlorophyllous areas and non-green areas dispersed on the leaf. Such a plant is allowed to starve in dark for 24 to 48 hours to deplete them of starch.
An outline of a leaf from this plant is drawn on a paper and areas which are green and non-green are traced on it. Now the plant is exposed to light for 24 to 48 hours. After this the variegated leaf is tested for starch by iodine.
Observation:
It is seen that the presence of starch is shown only in green areas and not in non-green areas.
Inference:
This experiment shows that photosynthesis can occur in leaf in its green areas only containing chlorophyll and without chlorophyll no photosynthesis takes place.
Experiment # 5
To Study Environmental Factors Controlling Photosynthesis:
(a) Light factor:
(i) To study the effect of light intensity:
Experiment:
An experiment is set up as in Expt. 1 (a) or 1 (b). The set-up is placed at a distance of two meters from a strong artificial light (200-watt) without a reflector. The number of bubbles coming out per minute is counted. The distance between the set-up and the light source is reduced by one-eighth and the number of bubbles per minute is recorded.
A few minutes should be allowed for adjustment to a particular light intensity by the plants. The distance is again reduced by one-fourth and one-half respectively of the original one and number of bubbles is recorded similarly in each case. The temperature should be carefully recorded every time.
Results:
The number of bubbles is plotted against the respective light intensity, keeping in mind that light intensity varies inversely as a square of the distance. .The light intensity at the maximum distance may be measured, or it may be taken as unity, and the respective intensities of the shorter distances are calculated.
Discussion:
Generally the rate of photosynthesis is increased if the intensity of light is increased until some other factors, e.g., CO2 conc., temperature, etc., become limiting. Within a limited range of intensity, the photosynlhetic rate is more or less proportional to the light intensity, if die CO2 concentration is not limiting.
At high light intensity, the rate of photosynthetis is inhibited (solarization due to photo-oxidation). At a very low light intensity stomata are closed and hence entrance of CO2 is checked and consequently the rate of photosynthesis decreases.
Again as a result of very high light intensity transpiration rate increases causing reduction of water content of the cells of the leaf and thereby retarding photosynthetic rate. Moreover at high light intensity photo-oxidation of chlorophyll takes place. Within a limited range, increase in light intensity also increases temperature which does not, therefore, become a limiting factor.
(ii) To study the effect of light quality:
Experiment:
The effect of light quality on the rate of photosynthesis can be studied by the same experimental set-up, keeping the light source at a constant distance and by separately using blue, green and red coloured cellophane papers as filter. The numbers of bubbles are counted in each case.
Results:
The results are plotted graphically taking quality of light as abscissa and number of bubbles as ordinate.
Discussion:
The effects of different light qualities are different on the rate of photosynthesis. The rate of photosynthesis is maximum at the red region of visible spectrum (655 nm) and secondary maximum in the blue region (440nm). Thus two peaks arc obtained, while a plateau is formed at the green region because most of light of this quality is reflected by the plant.
The action spectrum thus shows that the maximum efficiency in photosynthesis is exhibited by the- red and blue regions of the visible spectrum (400 to 700 nm).
(b) Carbondioxide factor:
Experiment:
An experiment is set up as in Expt. 1(a) or 1(i) using 500 ml of previously boiled and cooled distilled water. Now six lots of 1.25 gm weights of KHGO3 are taken separately. The set-up is placed in bright light.
A graduated tube or a centrifuge tube filled with water is inverted over the jet. 125 gm of KHGO3 then added and allowed to dissolve completely. After waiting for 5 minutes, the volume of oxygen collected in the graduated tube is recorded.
The remaining five weights of bicarbonate are separately added one by one allowing time to dissolve the bicarbonate completely and volume of O2 collected after each addition of bicarbonate is recorded.
After addition of each lot of KHGO3 about 5 minutes should be allowed for complete dissolution and then the volume of O2 is collected for a specific duration. The concentrations of the solution, after addition of first to sixth lot of KHCO3 packets, will be 0.25, 0.50, 0.75, 1.0, 1.25 and 1.50 per cent respectively.
Results:
The results are graphically plotted taking concentration of bicarbonate as abscissa and volume of O2 as ordinate.
Discussion:
An increase in the CO2 concentration of the surrounding atmosphere results in an increase in the rate of photosynthesis until a point is reached where further increase in CO2 concentration brings about no increase of photosynthetic rate. At this stage other factors like light or temperature become limiting.
Again high CO2 concentration retards photosynthesis because of:
(i) Increased acidity (HCO3 from bicarbonate and H+ from water forming H2CO3) of the mesophyll cells,
(ii) Of a narcotic effect on the metabolic function of the cells, and (m) closing of stomata.
N.B. In case of terrestrial plants effect of CO2 concentration on the rate of photosynthesis (in terms of starch formation) may be studied by covering the plant material with a bell jar fitted with a CO2-gas-measuring burette at the top to control the CO2 inflow inside the belljar with respect to time.
(c) Temperature factor:
Experiment:
The same procedure and set-up employed in Expt. 3(a) can be used for this experiment’. In this experiment the temperature of -water is raised by 5°C. Instead of 10°C. Number of bubbles per unit time is recorded at each temperature. Readings should be taken in at least five different sets of temperatures (range of temperature may be kept between 10°G. to 50°C.).
Results:
Results are graphically plotted taking temperature as abscissa and rate of photosynthesis as ordinate.
Discussion:
Photosynthesis is restricted to a temperature range (0°C. to 60°C.) that roughly corresponds to that tolerated by protein compounds. Although the photochemical part of photosynthesis is independent of temperature, biochemical part, which is controlled by enzyme activity, is strictly temperature dependent.
However, there appears to be a wide variation in adaptability amongst plants in their ability to tolerate temperature extremes. Generally above 35°C temperature has a deteriorating effect.
N.B. 0.10 may be calculated by knowing the rate of photosynthesis at every 10°C rise of temperature from the graph. If necessary the graph may be extrapolated.
Experiment # 6
To Study Blackman’s Law of Limiting Factors (Interaction of Environmental Factors):
Experiment:
Experiment is set up as in Expt. 5(i) keeping light intensity and temperature constant. The concentration of CO2 is increased until the rate of photosynthesis becomes constant. Now the intensity of light is increased and the rate of photosynthesis is measured until again a constant level is reached. Then the temperature of water is increased and the rate of increase in photosynthesis is noted.
Results:
The results are graphically plotted as shown in the model graph (Figure 30).
Discussion:
The rate of photosynthesis at a constant temperature and light intensity increases with increasing concentration of CO2 up to a certain limit when light or temperature becomes limiting. At this stage increase in light intensity, keeping other two factors constant again increases the rate to a point when temperature becomes a limiting factor. Increase in temperature up to a limit hastens the rate of photosynthesis.
Under a given set of conditions the rate of a process may, therefore, be largely controlled by one or two of the limiting factors and not by all the essential factors. This principle is called Blackman’s law of limiting factor since he first proposed it. This is evident from the above experiment.
Experiment # 7
To Study on the methods of Measuring the Rate of Photosynthesis:
(a) Rate of photosynthesis in aquatic plants:
Experiment:
Rate of photosynthesis may be measured by counting number of bubbles per unit time (Refer Expt. 1.a or 1.6) or by measuring the volume of O2 evolved per unit time (Refer Expt. 5.b).
N.B. The rate may also be measured by Winkler’s titration procedure which gives the mg or ml of O2 evolved (i.e., remains in dissolved condition) by constant amount of plant material per hour.
(b) Rate of photosynthesis in Terrestrial plants with the help of Ganong’s photosynthometer:
Experiment:
Ganong’s photosynthometer is a standard apparatus by which an estimation of amount of CO2 absorbed at a given time during photosynthesis by green plants can be made. With accurate handling, the amount of O2 evolved during photosynthesis can also be measured by this apparatus and an idea about photosynthetic quotient O2CO2 can be Stopcock/obtained.
The apparatus essentially consists of three parts:
(a) A glass bulb fitted on a wooden base,
(b) A graduated tube with a stopcock, and
(c) A connecting link fitted with a stopcock which connects the graduated tube with the glass bulb. The volume of the whole set when fitted is 103 ml (Figure 31).
About 3 ml (measured by displacement of water) of the experimental material (any suitable fresh green leaf) is placed in the glass bulb. The volume of the apparatus now becomes 100 ml. The graduated tube is now inverted; its stopcock is closed and filled with water up to a desired mark. The graduated tube is then fitted with the connecting link.
Now stopcock of the connecting link (c) is closed and its other hollow end is filled with water. It is then inverted into a suitable measuring cylinder containing water closing the hollow end with the palm. The set is now clamped keeping the level of water in the measuring cylinder up to the hole of the connecting link (h).
Now the top of the graduated tube is connected to a CO2 generator or Kipp’s apparatus. Both the stopcocks of the graduated tube and the connecting link are open and CO2 is admitted into it. The water in the graduated tube gradually flows down and the tube is filled up by CO2The-stopcock of the adulated tube is closed when the level of water in the graduated tube falls to the level of water outside.
The tube now contains desired amount of CO2 the whole set is now fitted at the top of the glass bulb (a), so that the hole of the connecting link coincides with that of the glass bulb. This ensures that the pressure inside the bulb and the hollow end of the connecting link is at atmospheric pressure.
The connecting link is then slightly twisted to cut off the connection with the atmosphere. All connections are then made air-tight. Now the stopcock of the connecting link is opened to allow CO2 to diffuse into the bulb-the whole apparatus is kept in bright sunlight for about three hours. After noting the time the stopcock of the connecting link is dosed and the graduated tube is taken out from the bulb.
It is then put in a basin of water and the connecting link is removed under water. The graduated tube is fixed with the zero mark exactly on the surface of water and the stopcock of the graduated tube is gently opened to allow water to rise inside the tube up to the zero mark.
Now one test tube is completely filled with 30% KOH solution and is fitted to the top of the graduated tube with the help of rubber tubing which is fitted with a pinch cock. The tube is removed from water, pinch cock is opened and the KOH solution is allowed to flow into the graduated tube.
It is shaken thoroughly, KOH solution is drained back into the test tube and the pinch cock is closed. The end of the graduated tube is then placed under water with its zero mark at the surface level and the test tube is disconnected closing the stopcock of the graduated tube.
The water now rushes up to a mark corresponding to the volume of CO2 absorbed by KOH which equals the volume of CO2 left unutilised by the photon synthetic experimental material after the desired time.
The test tube is now filled with alkaline pyrogallate solution and the procedure is repeated to measure the volume of O2 evolved. The difference between the actual amount of CO2 present at the beginning of experiment and the amount of unused CO2 gives the actual amount of CO2 utilised in photosynthesis. The photosynthctic quotient (O2/CO2) may be calculated from the results.
The rate of photosynthesis is expressed as ml of CO2 absorbed or O2 evolved per gram weight of the experiment material per hour.
N.B. The experiment is somewhat erroneous because of the following reasons:
(i) The volume of CO2 left inside the bulb as well as in the hollow end of the connecting link is neglected,
(ii) The measurement of volume of O2 evolved in photosynthesis is not accurate because this disregards the volume of O2already present in the atmosphere inside the apparatus (air contains about 16% O2), and
(iii) CO2 is slightly soluble in water.
(c) Rate of photosynthesis in terrestrial plants by measuring dry weight increase or net assimilation:
Experiment:
Several weighing bottles are dried in an oven at 100°C., cooled in a desiccator and accurately weighed. These are stored in a desiccator until ready for use. Bottles arc serially marked. Two suitable potted plants are kept in a dark room for a day before the experiment is started.
The following morning at a convenient hour one potted plant is transferred to a sunny place and 50 discs are cut out from the leaves with a cork borer of 1 cm diameter.
During cutting of discs, the leaf may be supported against a cork or rubber stopper. A representative sampling of all the leaves on the plant is taken except the very young and senescent leaves. The samples are taken in weighing bottles and fresh weights and dry weights are determined.
The plant from which the discs have been collected is allowed to remain in the sunny place for at least four hours. The duration of experiment, temperature and light intensity are recorded. The plant is adequately watered during these periods.
After four hours the discs are similarly cut out, taken in weighing bottles, fresh and dry weights are determined. The experiment is repeated at an interval of four hours as long as time permits. The same procedure is repeated in case of plants kept in dark.
Results:
Increase in dry weight per four hours by 50 discs is calculated in each case and the result is expressed as mg dry weight increase per sq. cm of the leaf per hour.
N.B. The amount of CO, consumed per hour per sq. cm may be calculated, assuming all the photosynthate as glucose, as follows.
Again the number of litres of air required to supply the CO2 absorbed per sq. cm per hour may also be calculated as follows. If amount of CO2 consumed/hr/sq. cm of leaf is taken as X, volume of CO2 in air as 0.03 per cent and weight of 1 ml of CO2 as 1.977 mg at standard conditions then the amount of air is ![]()
Experiment # 8
Determination of Real and Apparent Photosynthesis:
Experiment:
Two experimental set-ups are arranged as in Expt. 2.a (iv). Here tap water is used (which may be previously oxygenated by photosynthesis). The experimental materials should be of comparable number and weight in each case.
The initial O2 content of water in each set-up is determined following the procedure given in the referred experiment. One set-up is kept in bright light for photosynthesis and the other in dark for respiration only. Alter two hours the O2 content of water of both the set-ups is again determined.
Results:
The difference between the final and the initial content of O2 in case of the set-up kept in light gives the excess of O2 liberated by photosynthesis. This amount indicates apparent photosynthesis.
Again the difference between the initial and final content of O2 in case of the set-up kept in dark gives the amount of O2 consumed during respiration. When this amount of O2 is added to the amount of O2 liberated by photosynthesis it gives the real photosynthesis.
Discussion:
When the net result of photosynthesis is measured and no correction is made for respiration it is called the apparent pkolosytahesis. When, on the other hand, corrections for respiration are made, the photosynthesis thus calculated is called die real photosynthesis or true photosynthesis.
So to obtain a measure of true photosynthesis it is desirable to make corrections for respiration. Some of the O2 given off in photosynthesis is used up in respiration and some of the CO2 given off in respiration is taken up in photosynthesis.
It is therefore necessary to measure the respiration of an identical sample in dark. It is easily seen that the apparent photosynthesis is less than the true photosynthesis by the amount of O2 used up in respiration.
The respiration error is variable and is always present, and it thus complicates attempts to measure true photosynthesis, since the factor which influence photosynthesis are likely also to influence respiration.
Experiment # 9
To Show That the Entry of CO, Takes Place Through Stomatal Pores:
Experiment:
A suitable potted plant is so selected that its leaves are dorsiventral and upper surfaces are thickly circularised. The plant is kept in dark for two days to deplete their leaves of starch. Now some leaves are smeared with Vaseline only on the lower surfaces and other leaves are smeared only on the upper surfaces.
Vaseline should be applied as a thin film so that stomatal pores are closed. The plant is then kept in light for several hours. After that period the leaves are severed and tested for starch by iodine.
Observation:
It is observed that the leaves having Vaseline applied on the lower surface only do not show the presence of starch whereas the leaves in which Vaseline was applied on the upper surface shows the presence of starch by iodine.
Inference:
In case of the leaves smeared with Vaseline on the lower surface starch synthesis cannot take place because CO2 could not enter into the leaf for use in photosynthesis owing to the closure of stomatal pores by Vaseline.
Those leaves in which Vaseline has been applied on the upper surface have their stomata open on lower surface through which CO2 could enter and allow the photosynthesis to occur resulting in starch synthesis. Thus the experiment shows that the CO2 enters the leaves through stomatal pores of the lower surface in case of dorsiventral leaves.
Experiment # 10
To Show the Necessity of Light for Synthesis of Chlorophyll in Leaves:
Experiment:
Some pea or gram seeds are allowed to germinate in dark as well as in light.
Observation:
After 10 days the dark grown seedlings show yellow or white leaves and stems. Other seeds grown in light show normal seedlings with green leaves and stems. When etiolated plants are exposed to light these turn to green.
Inference:
The experiment shows that light is necessary for chlorophyll synthesis. In a light-mediated reaction protochlorophyll is reduced to form chlorophylla. Photoreduction of protochlorophyllide to chlorophyllide a, followed by phytol esterification to form chlorophyll a, is now thought to be the major pathway.
Experiment # 11
To Show that Starch Synthesis In Etiolated (Or Albino) Leaves are Independent of Light Provided Sugars are Present:
Experiment:
Two comparable leaf samples are picked from etiolated rice or wheat seedlings grown in a dark room. Each sample is placed in a beaker of 100 to 150 ml capacity and some weight is placed on the leaves to prevent them from floating.
One sample is covered with pure water and the other with 0.1 M sucrose solution for two days in the dark room. The samples are removed from the beaker; excess liquid is blotted off, placed on moistened filter paper in petridishes and set aside in the dark room for a period of 24 hours. At the end of the experimental period the leaves from both the sets are tested for starch by iodine.
Observation:
No starch is obtained in case of leaves kept in water whereas starch synthesis takes place in case of leaves kept in sucrose solution.
Inference:
The experiment shows that the starch synthesis in etiolated leaves is independent of light if sugars arc supplied to the etiolated leaves.