ADVERTISEMENTS:
The below mentioned article includes a list of three experiments on proteins.
1. Experiment to localize proteins in a cell:
Requirements:
Fresh tissues, razor, acid fuchcin, acetic acid, glycerine jelly, slides, cover slips.
ADVERTISEMENTS:
Method:
1. Cut the thin sections of fresh tissues with the help of razor, put them on slide and incubate them in 0.005% acid fuchcin in 1% acetic acid for 10 minutes.
2. Rinse the sections and mount them in glycerine jelly.
Observations and results:
ADVERTISEMENTS:
Observe under the microscope. Proteins present in the cells stain red.
2. Experiment to perform colour tests of proteins:
What are proteins?
Proteins are the substances made of one or more polypeptides, which themselves are made of amino acids. There are many different kinds of proteins, each with its own sequence of amino acids. An amino acid is a class of organic compounds with a carboxyl group (-COOH), an amino group (NH2) and a “side group”, all attached to a central carbon atom. About 20 different amino acids are found in proteins.
1. Xanthoproteic Test:
Principle:
It is based on the fact that heating a protein with nitric acid produces a yellow colour that turns orange on addition of alkali.
Requirements:
Protein solution (or gram flour, legumes or soya bean seeds or flour), test tube, HN03, alkali spirit lamp.
Method:
ADVERTISEMENTS:
1. Take 2-3 ml of protein solution in a test tube and add 1ml of conc. nitric acid. A white precipitate is formed. In case of legumes or soya bean, make their suspension in water and follow the same procedure.
2. Heat the test tube on a sprit lamp. White precipitate changes into yellow and ultimately the solution becomes yellow-coloured.
3. This yellow colour turns orange on addition of alkali.
(Presence of benzene ring is responsible for the yellow colour in the test. Aromatic amino acids, especially tryptophan and tryosine are responsible for this test).
ADVERTISEMENTS:
2. Biuret test:
Principle:
This is based on the fact that the violet colour appears when a protein or tripeptide is treated with sodium hydroxide and dilute copper sulphate.
Requirements:
ADVERTISEMENTS:
Test tube, protein solution (or gram flour, legumes or soya bean), Biuret reagent.
Method:
1. Take 1.5 ml of a solution containing 0.25-2.0 mg. of protein in a test tube. (In case of legumes or soya beans make their suspension in water).
2. Add 1.5 ml of Biuret reagent and keep the test tube in the test tube stand for about 30 minutes at room temperature. Violet colour is produced.
ADVERTISEMENTS:
(The colour is due to the formation of a complex of cupric ions with one or two peptide bonds and of many other kinds of structures. This name to the test is even due to the formation of colour similar to that formed between copper and biuret, i.e., -H2NCONHCONH2).
3. Millon’s Test:
Principle:
It is based on the fact that many phenols yield red colour or precipitate when treated with an acid solution of mercuric, nitrous and nitrate ions. Proteins show this reaction due to the normal presence of tryosine.
Requirements:
Protein solution (or gram flour, legumes or soya bean), test tube, Millon’s reagent.
ADVERTISEMENTS:
Method:
1. Take 5 ml of protein solution or the suspension of gram flour, legumes or soya bean in water in a test tube.
2. Add 2 to 3 drops of Millon’s reagent (10% mercuric sulphate in 10% sulphuric acid, called first solution; or mercury, conc. nitric acid and water in the proportion of 1 : 2 :4, called second solution).
3. Heat the mixture. If the first solution is used in the preparation of Millon’s reagent, a clump of proteins is formed. It may also turn into red colour. If the second solution is used, a precipitate is formed which may turn reddish on further heating.(Proteins show this reaction due to the normal presence of tryosine).
Observations:
Tabulate your observations and results in the form of following Table 2.1:
ADVERTISEMENTS:
3. Experiment to determine total protein in food samples by Micro- Kjeldahl method:
Principle:
Protein content present in foodstuff samples is determined by evaluating the nitrogen content by any of the methods. After that the protein percentage in the sample is calculated by multiplying the factor 6.25 for foodstuffs like maize, sorghum, pulses and millets. 5.95 for nee and 5.7 for wheat.
Total protein is calculated by Micro-Kjeldahl method discussed below:
Micro-Kjeldahl Method:
This method of determination of protein consists in the digestion of foodstuff sample in concentrated nitrogen-free H2SO4 which dehydrates and oxidises.
The carbon in the sample is oxidised due to following reaction:
2H2SO4 + C = CO2 + 2H2O + 2SO2
The nitrogen present in foodstuff sample is transformed into ammonia which forms ammonium sulphate. This ammonia is liberated subsequently by roiling with alkali and absorbed by standard hydrochloric acid.
In this method, the boiling point of sulphuric acid is raised by adding small globule of mercury and potassium sulphate to oxidise the organic substances of the foodstuff sample. Actually, mercury promotes conversion of organic nitrogen to ammonia, and CuSO4 acts as a catalyst.
Requirements:
Samples of foodstuffs (e.g., pulses sorghum, maize, millets, rice, wheat), digestion flask, catalyst mixture (made up of 99.0 gm. K2SO4,4.1 gm. HgO and 0.8 gm. of CuSO4, all ground well in a mortar), N-free concentrated sulphuric acid, burner, water, pipette, boric acid solution (made by dissolving 4 gm. of boric acid in some warm distilled water and dilute to 100 ml Erlenmeyer flask, methyl-red-bromocresol green indicator mixture (prepared by mixing 1 part of 0.2% methyl red in ethanol with 5 parts of 0.2% bromocresol green in ethanol), distillation unit, sodium hydroxide-sodium thiosulphate solution (prepared by dissolving 50 gm. of NaOH and 5 gm. of Na2S2O3.5H2O in distilled water and volume made up to 100 ml), receiving flask, hydrochloric acid solution 0.02 N, Micro-kjeldahl distillation unit, Micro-kjeldahl digestion assembly.
Method and observations:
1. Weigh 40 mg of the given sample of foodstuff and transfer it to a digestion flask.
2. Add in it 1gm of catalyst mixture and 2 ml of concentrated sulphuric acid.
3. Digest the mixture first in low flame and then in high temperature (upto about 370°C) till it becomes colourless. The digestion process completes in about 45 minutes.
4. Cool the entire mixture, add small quantity of water and shake well till all the solids are dissolved.
5. Now pipette 6 ml of boric acid solution in a 100 ml Erlenmeyer flask, add in it 2 – 3 drops of indicator solution and keep the flask under condenser.
6. Now transfer the digest into the distillation unit and thoroughly rinse the digestion flask 3-4 times adding 2-3 ml distilled water each time.
7. Add 8 ml of sodium hydroxide and sodium thiosulphate solution to still and steam distill it un-till about 20 ml of distillate collects.
8. Now lower the receiving flask and continue distillaion for one more minute and then remove the receiving flask.
9. Now start titration and titrate the contents of the receiving flask to grey end point or till the appearance of violet colour.
10. Make blank determination (i.e., without sample) by using all the reagents as used earlier and calculate the nitrogen percentage.
Result:
Nitrogen percentage can be calculated by the following formula:
Protein percentage may be calculated by the formula 6.25 or 5.95 or 5.76 etc. representing the factor for the given sample of foodstuff.
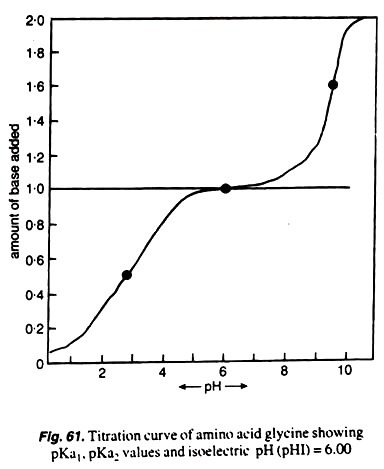
Standard Curve:
Amino acids (e.g., glycine, tryptophane, tyrosine etc.) are ionized to various degrees, and it depends mainly upon the ionizable groups they possess. The dissociable constants are called pK values and these are determined for the ionizablegroups.
If a solution of glycine hydrochloride (0.2 M glycine solution with 0.2 MHCl) is titrated with alkali (0.1 M NaOH) solution and pH of solution is noted at each titration, the isoelectric pH of this amino acid can be determined.
The first pK1 value is taken when the COOH group is ionized, and the second pK2value is taken or observed when ammonium ion is dissociated. All these results can be plotted graphically in the form of a curve called biphasic curve (Fig.61).