ADVERTISEMENTS:
Read this essay to learn about Plant tissue Culture. After reading this essay you will learn about: 1. Definition of Plant Tissue Culture 2. History of Plant Tissue Culture 3. Basic Requirements 4. General Techniques 5. Basic Aspects 6. Cellular Totipotency 7. Differentiation 8. Methods in Plant Tissue Culture 9. Applications of Plant Tissue Culture 10. Morphogenesis 11. Subculture or Secondary Cell Culture and Others
Essay on Plant Tissue Culture Contents:
- Essay on the Definition of Plant Tissue Culture
- Essay on the History of Plant Tissue Culture
- Essay on the Basic Requirements of Plant Tissue Culture
- Essay on the General Techniques of Plant Tissue Culture
- Essay on the Basic Aspects of Plant Tissue Culture
- Essay on the Cellular Totipotency
- Essay on the Differentiation
- Essay on the Methods in Plant Tissue Culture
- Essay on the Applications of Plant Tissue Culture
- Essay on the Morphogenesis
- Essay on the Subculture or Secondary Cell Culture
- Essay on the Soma-Clonal Variation
- Essay on the Somatic Hybrids and Cybrids
- Essay on the Micro-Propagation
- Essay on the Artificial Seed
- Essay on the Cryopreservation
Essay # 1. Definition of Plant Tissue Culture:
Plant tissue culture has a great significance in plant biotechnology specially in the crop improvement programmes. The term tissue culture may be defined as the process of in-vitro culture of explants (pieces of living differentiated tissues) in nutrient medium under aseptic conditions. However, in general, the tissue culture includes the term tissue culture as well as cell culture, organ culture and suspension culture also.
Plant tissue culture is fundamental to most aspects of biotechnology of plants. It is evident now that plant biotechnology is one of the most beneficial of all the sciences. The products of plant biotechnology are being transferred rapidly from laboratories to the fields.
ADVERTISEMENTS:
Also, the plant tissue culture has become of great interest to the molecular biologists, plant breeders and even to the industrialists, as it helps in improving the plants of economic importance. In addition to all this, the tissue culture contributes immensely for understanding the patterns and responsible factors of growth, metabolism, morphogenesis and differentiation of plants.
Related Terms:
Tissue Culture:
The in-vitro culture of the tissue e.g. Callus culture
Cell Culture:
ADVERTISEMENTS:
Denotes the in-vitro culture of single or a few cells.
Organ Culture:
This term is used for in-vitro culturing of organs like embryo, root or shoot apices.
Suspension Culture:
Defined as the culture of cell and cell aggregates suspended in a liquid medium.
Ex plant:
The excised piece of differentiated tissue or the organ which is used for culture is called as explant. e.g., embryos, young leaf, bud, etc.
Callus:
The undifferentiated mass of cells is referred to as callus. The cells of callus are meristematic in nature.
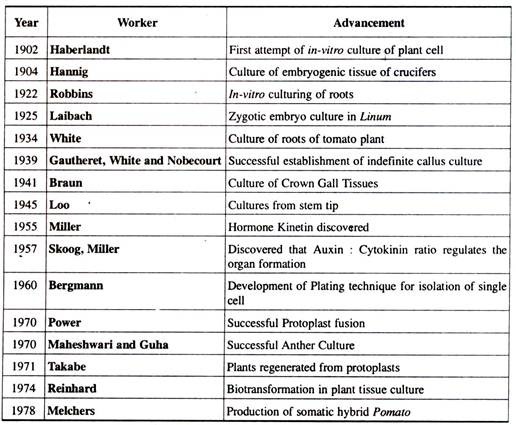
Essay # 2. History of Plant Tissue Culture:
G. Haberlandt, a German botanist, in 1902 cultured fully differentiated plant cells isolated from different plants. This was the very first step for the beginning of plant cell and tissue culture. Further contributions were made by the Cell Doctrine which admitted that a cell is capable of showing totipotency.
ADVERTISEMENTS:
With the identification of a variety of chemicals like cytokinin, auxin, other hormones, vitamins, etc. and their role in affecting cell division and differentiation, the methods of plant tissue culture developed in a proper manner. Three other scientists Gautheret, White and Nobecourt also made valuable contributions to the development of plant tissue culture techniques.
Later on, a number of suitable culture media were developed, for culturing plant cells, tissues, protoplasts, embryos, anthers, root tips, etc. The discovery and understanding of role of plant growth hormones in the multiplication of cell also provided an extra aid for the development of in-vitro culture methods of plants.
The first plant from a mature plant cell was regenerated by Braun in 1959. Foundation of commercial plant tissue culture was laid in 1960 with the discovery for a million fold increase in the multiplication of Cymbidium (an orchid) which was accomplished by G.M.Morel.
ADVERTISEMENTS:
In India, the work on tissue culture was initiated during 1950s at University of Delhi. This initiation is credited to Shri Panchanan Maheshwari who was working there in the Department of Botany. Discovery of haploid production was a land-mark in the development of in-vitro culturing of plants.
Shri S.C. Maheshwari and Sipra Guha made a remarkable contribution in the development of plant tissue culture in India. Later on the development in the composition of nutrient media and genetic engineering served as a basis for further success in the plant tissue culture techniques.
Gottleib Haberlandt was the first person to make attempts for plant tissue culture, i.e., he developed the concept of in-vitro culture of plant cells and is aptly regarded as the father of tissue culture. Thereafter, there happened some dramatic advances in tissue culture techniques.
Some of the early classical contributions in the field of plant tissue culture are tabulated below:
Essay # 3. Basic Requirements and Techniques of Plant Tissue Culture:
The main requirements of plant tissue culture are:
(1) Laboratory Organisation
(2) Culture Media
(3) Aseptic Conditions
1. Laboratory Organisation:
ADVERTISEMENTS:
In a standard tissue culture lab, there must be a few basic facilities like:
i. A Media Room for preparation, sterilization and storage of culture media.
ii. Facilities for washing of lab-wares, explants, etc.
iii. Space for storage of lab-wares.
iv. Culture rooms or incubators where conditions of temperature, humidity and light etc. can be maintained.
v. Observation and Data Collection area.
2. Culture Media:
ADVERTISEMENTS:
The formulation or the medium on which the explant is cultured is called culture medium. It is composed of various nutrients required for proper culturing. Different types of plants and organs need different compositions of culture media. A number of media have been devised for specific tissues and organs.
Some important of them are:
MS (Murashige and Skoog) Medium
LS (Linsmaier and Skoog) Medium
B5 (Gamborg’s) Medium
White’s Medium, etc.
ADVERTISEMENTS:
Important constituents of a culture medium are:
Organic supplements:
(a) Vitamins like thiamine (B1), Pyridoxin (B6), Nicotinic Acid (B3), etc.
(b) Antibiotics like Streptomycin, Kanamycin;
(c) Amino Acids like Arginine, Asparagine.
(ii) Inorganic Nutrients:
Micronutrients as Iron (Fe), Manganese (Mn), Zinc (Zn), Molybdenum (Mo), Copper (Cu), Boron (B).
Macronutrients include six major elements as Nitrogen (N), Sulphur (S), Phosphorus (P), Potassium (K), Calcium (Ca), Magnesium (Mg).
(iii) Carbon and Energy Source:
Most preferred carbon source is Sucrose. Others include lactose, maltose, galactose, raffinose, cellobiose, etc.
(iv) Growth Hormones:
a. Auxins-mainly for inducing cell division.
b. Cytokinins-mainly for modifying apical dominance and shoot differentiation.
c. Abscisic Acid (ABA)-Used occasionally.
d. Gibberellins-Used occasionally.
Gelling Agents:
These are added to media to make them semisolid or solid. Agar, Gelatin, Alginate etc. are common solidifying or gelling agents.
Other Organic Extracts:
Sometimes culture media are supplemented with some organic extracts also like coconut milk, orange juice, tomato juice, potato extract, etc.
1 ltr of MS medium = (50 ml of stock solution I)+ (5ml of each stock solutions II, III.IV)
3. Aseptic Conditions:
Maintenance of aseptic conditions is the most critical and difficult aspect of in-vitro culturing experiments. Aseptic condition mean the conditions free from any type of microorganisms (so as to prevent the loss of experiment by contamination). For this, sterilization (i.e., complete removal or killing of microbes) is done. The most common contaminants in culture are fungi and bacteria.
Measures to be taken for maintaining asepsis during tissue culture are:
i. Sterilization of the culture vessels using detergents, autoclaves, etc.
ii. Sterilization of instruments like forceps, needles etc. by flame sterilization.
iii. Sterilization of culture medium using filter sterilization or autoclaving methods.
iv. Surface sterilization of explants using surface disinfectants like Silver Nitrate (1%), H2O2 (10-12%), Bromine water (1-2%), Sodium Hypochlorite solution (0.3-0.6%), etc.
The whole procedure of plant tissue culture is to be carried out essentially under aseptic conditions. So, the overall design of the laboratory must focus on the maintenance of aseptic conditions. Secondly, the worker is also required to have proper knowledge of operating various equipment’s like pH meter, balance, laminar air flow, microscope, etc.
While performing the tissue culture experiments there must present the first aid kits and fire extinguishers in the laboratory to avoid any mishap or accident. In addition, proper attention should be given while handling the toxic chemicals and all the chemicals should be kept in correct labeled containers and bottles.
Essay # 4. General Technique of Plant Tissue Culture:
General technique of plant cell, tissue and organ culture is almost the same with a little variation for different plant materials. There are certain basic steps for the regeneration of a complete plant from an explant cultured on the nutrient medium (Fig. 1).
These basic steps for in-vitro culturing of plants are:
(a) Selection and Sterilisation of Explant:
Suitable explant is selected and is then excised from the donor plant. Explant is then sterilized using disinfectants.
(b) Preparation and Sterilisation of Culture Medium:
A suitable culture medium is prepared with special attention towards the objectives of culture and type of explant to be cultured. Prepared culture medium is transferred into sterilized vessels and then sterilized in autoclave.
(c) Inoculation:
Sterilized explant is inoculated (transferred) on the culture medium under aseptic conditions.
(d) Incubation:
Cultures are then incubated in the culture room where appropriate conditions of light, temperature and humidity are provided for successful culturing.
(e) Sub culturing:
Cultured cells are transferred to a fresh nutrient medium to obtain the plantlets.
(f) Transfer of Plantlets:
After the hardening process (i.e., acclimatization of plantlet to the environment), the plantlets are transferred to green house or in pots.
Equipment in Tissue Culture Lab:
Essay # 5. Basic Aspects of Plant Tissue Culture:
In plant tissue culture technique, an explant is taken, it is cultured on a nutrient medium under certain conditions and finally we obtain a whole new plant. How does it happen?
The answer to this question lies in the inherent capacities of plant cells that are differentiation and cellular totipotency.
Essay # 6. Cellular Totipotency:
The potential of a plant cell to grow and develop into a whole new multicellular plant is described as cellular totipotency. In other words, the property of a single cell for differentiating into many other cell types is called as totipotency. This is the property which is found only in living plant cells and not in animal cells (exception being stem cells in animals). The term totipotency was coined in 1901 by Morgan. During culture practice, an explant is taken from a differentiated, mature tissue. It means, the cells in explants are generally non-dividing and quiescent in nature.
To show totipotency, such mature, non-dividing cells undergo changes which revert them into a meristematic state (usually a callus state). This phenomenon of reverting back of mature tells to dividing state is called dedifferentiation. Now, these dedifferentiated cells have the ability to form a whole plant or plant organ. This phenomenon is termed as re-differentiation.
Dedifferentiation and re-differentiation are the two inherent phenomena involved in the cellular totipotency. Regarding this, it is clear that the cell differentiation is the basic event for development of plants and it is also referred to as cyto-differentiation.
To express its totipotency, a differentiated cell first undergoes the phenomenon of dedifferentiation and then undergoes the re-differentiation phenomenon (Fig. 3). Usually the dedifferentiation of the explant leads to the formation of a callus. However, the embryonic explants, sometimes, result in the differentiation of roots or shoots without an intermediary callus state.
Thus, from the above account it is clear that unlike animals (in which differentiation is irreversible usually), the plants have such a quality that even highly mature and differentiated cells have an ability to revert back to meristematic state. The property of totipotency of plant cells indicate that even the undifferentiated cells of a callus carry the essential genetic information required for regeneration of a whole plant.
It is also clear that all the genes responsible for dedifferentiation or re-differentiation are present within the individual cells and they become active for expression under adequate culture conditions. As totipotent cells are the basis of whole plant tissue culture techniques, so, by the exploitation of this potential of plant cells, biotechnologists are trying to improve the crop plants and other commercially important plants.
Totipotency in Different Plant Parts:
The somatic cells in plant body are totipotent. It is to be noted here that only the living plant cells have the ability to regenerate and the dead cells which lack cytoplasm and nucleus (tracheid’s, vessel elements, etc.) are not totipotent at all.
Different plant parts have different totipotent abilities. For example, in tobacco plant, the type of bud formed by in-vitro culture of the epidermis of different regions of the plant are different in their form.
Another example to add here may be given about the totipotency of crown-gall cells which have the capacity to grow as an un-organised mass of cells under normal conditions, however whole plants can be recovered from them in culture. Thus, it is clear that totipotency is not similar in all plant parts.
Applications of Totipotency:
Cellular totipotency of plants cells has proved to be a boon to mankind as it is the basis of plant tissue culture. The plant tissue culture exploits this unique property of plant-cells to attain commercial benefits.
Various applications of cellular totipotency are:
i. It has potential applications in the crop plant improvement.
ii. Micro-propagation of commercially important plants.
iii. Production of artificial or synthetic seeds.
iv. It helps in conservation of germplasm (genetic resources).
v. This ability is utilized for haploid productions.
vi. Applied in producing somatic hybrids and cybrids.
vii. Helps in cultivation of those plants whose seeds are very minute and difficult to germinate.
viii. Also helps to study the cytological and histological differentiations.
ix. For high scale and efficient production of secondary metabolites.
x. The genotypic modifications can also be possible.
Essay # 7. Differentiation:
While studying totipotency, it is stated that the dedifferentiation and redifferentiation processes result in the differentiated plant organs, finally producing a whole plant. In case of plants, the differentiation is reversible but in animals, it is irreversible.
The term differentiation describes the development of different cell types as well as the development of organised structures like roots, shoots, buds, etc., from cultured cells or tissue.
Differentiation may also be defined in simple words as the development change of a cell which leads to its performance of specialised function. However, normally morphological characteristics. For example, differentiation accounts for the origin of different types of cells, tissues and organs during the formation of a complete multicellular organism (or an organ) from a single-celled zygote.
Actually, the development of an adult organism starting from a single cell occurs as a result of the combined functioning of cell division and cell differentiation. Various techniques of tissue culture provide not only a scope of studying the factors governing totipotency of cells but also serves for the investigation of patterns and factors controlling the differentiation.
Types of Differentiation:
As stated earlier also, the plant cells have a tendency to remain in a quiescent stage which may be reverted to the meristematic stage. This process is termed as dedifferentiation and as a result of this, a homogeneous undifferentiated mass of tissue i.e., callus is formed. There callus cells then differentiate into different types of cells or an organ or an embryo.
On this basis, the differentiation may be of the following types:
(a) Cytodifferentiation
(b) Organ Differentiation
(c) Embryo Genic Differentiation
a. Cytodifferentiation:
The differentiation of the cells is an important event of the development of plants. The differentiation of different types of cells from the cultured cells is known as cytodifferentiation. When an undifferentiated callus re-differentiates into whole plant, it first undergoes cytodifferentiation.
Amongst different cytodifferentiations, the differentiation into vascular tissues has received maximum attention. However, it is important here to mention that the cells of mature xylem elements and phloem cells cannot be re-differentiated or cannot be reverted back to the meristematic state due to lack of cytoplasm in them.
Although, in initial stages of their development, they can be reverted to meristematic cells. Xylogenesis is the differentiation of parenchymatous cells (of callus) into xylem-like cells of vascular plants. Phloem differentiation is the formation of phloem-cells from parenchyma in culture.
Factors affecting cytodifferentiation:
(i) Physical factors like light, temperature and pH are effective at optimum levels.
(ii) Chemical factors.
a. Low Nitrogen content increases vascularization
b. High Ca++ ions stimulates the formation of tracheid’s and sieve tubes.
c. Sucrose in high concentration results in pronounced xylem differentiation.
(iii) Hormones:
Some hormones play important role in cytodifferentiation.
These are:
a. Auxin plays major role in vascularization.
b. Cytokinin promotes cytodifferentiation.
c. Gibberellins along with auxins promote it.
d. Abscisic acid inhibits it usually.
b. Organ Differentiation:
It is synonymous to organogenesis or organogenic differentiation. It refers to the development or regeneration of a complete organised structure (or whole plant) from the cultured cells/tissues (Fig. 4).
Organogenesis literally means the birth of organ or the formation of organ. It may occur either by shoot bud differentiation or by the formation of root. Organogenesis commences with the stimulus produced by the components of culture medium, the substances initially present in the original explants and also by the compounds produced during culturing.
Among different organs, which can be induced in plant tissue culture are included the roots, shoots, flower buds and leaves. Regenerations into flower buds and leaves occur in a very low frequency.
However, the roots and shoot bud regenerations are quite frequent. Out of all these types of organogenic differentiation, only the shoot bud differentiation can give rise to the complete plantlets therefore, it is of great importance in tissue culture practices.
The initiation of roots is termed as rhizogenesis while the initiation of shoots is called as caulogenesis and these two phenomena are affected by alterations in the auxin : cytokinin ratio in the nutrient medium. A group of meristematic cells called as meristemoids is the site of organogenesis in callus. Such meristemoids are capable of producing either a root or a shoot.
Organogenesis may occur either through callus formation or through the direct formation of adventitious organs (like adventitious shoot). Latter mode of organogenesis does not involve the intervening callus phase.
Shoot bud differentiation was first of all demonstrated by White (1939).
Further, in 1944, Skoog indicated that organogenesis could be chemically controlled. Shoot bud differentiation refers to the formation of shoot buds from the cultured cells by providing appropriate culture conditions and nutrient medium. The chemical and physical factors required for shoot bud differentiation vary for explants from different plant species.
Factors affecting organogenesis:
(i) Auxin: Cytokinin ratio in medium is an important factor affecting root/shoot bud differentiation in most plants.
(ii) Usually Gibberellic acid inhibits organogenesis.
(iii) Physiological state and size of explant play important role in organ differentiation.
(iv) Genotype of the donor plant plays a crucial role.
(iv) Physical factors like light, temperature, moisture, etc., play effective role in organogenesis.
c. Embryo Genic Differentiation:
The embryos formed from the somatic cells of plant in culture under in-vitro conditions are called as somatic embryos. When the somatic cells of plant organs result into the regeneration into embryos, then the process is called as somatic embryogenesis or embryo genic differentiation or embryogenesis (Fig. 5).
Somatic embryos are also referred to as embryoids, and they can be obtained either indirectly (with formation of callus) or directly from the explant without intervening callus formation. However, direct embryogenesis is not a normal process because the medium requirement for this is complex.
Somatic embryogenesis under in-vitro conditions was first of all observed by Steward et. al. (1958) in carrot (Daucus carota). Thereafter, somatic embryoids have been induced in many plants namely Citrus, Coffea, Zea mays, etc. To obtain embryoids, there is a requirement of two nutrient media, first for initiation and the other medium for proper development of the embryoid.
The development of somatic embryo passes through the stages like globular, heart-shaped, torpedo-shaped and finally giving rise to the cotyledonary stage of somatic embryo. A somatic embryo does not have any vascular connection with the explant or callus therefore it can be separated easily.
Somatic embryogenesis is not used very frequently for propagation of plants because, the technique is usually difficult and also, there is a high risk of occurrence of mutations. Another major drawback of somatic embryogenesis is that there are greater chances of loss of regenerative capacity on repeated sub-culturing.
Factors affecting Embryogenesis:
a. Physiological condition and type of explant.
b. Genotype of donor plant.
c. Growth regulators:
i. Auxin is essential for embryo initiation
ii. Cytokinin promotes embryogenesis
iii. Gibberellins inhibit embryo genic differentiation
iv. Abscisic Acid (ABA) suppresses it.
d. Nitrogen and Oxygen concentration
e. Physical factors like temperature and light.
Essay # 8. Methods in Plant Tissue Culture:
There are different methods of culturing plant material. These methods differ on the basis of explants used and their resultant products.
Some of the most popular and advantageous methods in plant tissue culture are discussed below:
1. Cell Culture:
Cell culture is actually, the process of producing clones of a single cell. The clones of cell are the cells which have been derived from the single cell through mitosis and are identical to each other as well as to parental cell. First attempts for cell culture were made by Haberlandt in 1902. However, he failed to culture single cell but his attempts stimulated other workers to achieve success in this direction.
The method of cell culture is meritorious over other methods of culturing because it serves as the best way to analyse and understand the cell metabolism and effects of different chemical substances on the cellular responses. Single cell culturing is of immense help in crop improvement programmes through the extension of genetic engineering techniques in higher plants.
The method of cell culture is done by following three main steps:
(a) Isolation of single cell from the intact plant by using some enzymatic or mechanical methods.
(b) In-vitro culturing of the single cell utilizing micro chamber technique, or micro drop method or Bergmann cell plating technique (Fig. 6).
(c) Testing of cell viability done with the phase contrast microscopy or certain special dyes.
It is important to note here that the cell cultures require a suitably enriched nutrient medium and it should be done in dark because light may deteriorate the cell culture. Large scale culturing of plant cells under in-vitro conditions provides a suitable method for production of large varieties of commercially important phytochemicals.
2. Suspension Culture:
A culture which consists of cells or cell aggregates initiated by placing callus tissues in an agitated liquid medium is called as a suspension culture. The continuous agitation of the liquid medium during a suspension culture is done by using a suitable device called as shaker, most common being the platform/orbital shaker.
Agitation with shaker is important because it breaks the cell aggregates into single cell or smaller groups of cells and it helps in maintaining the uniform distribution of single cell and groups of cells in the liquid medium.
A good suspension is the one which has high proportion of single cells than the groups of cells. Changes in the nutritional composition of medium may also serve as a useful technique for breakage of larger cell clumps (Fig. 7).
The general technique of suspension culture involves basically two types of cultures: batch culture and continuous cultures.
A batch culture is a suspension culture in which cells grow in a finite volume of the culture medium and as a result, medium gradually depletes. On the other hand, a continuous suspension culture is the one which is continuously supplied with nutrients by the inflow of fresh medium but the culture volume is normally constant.
3. Root Culture:
Pioneering attempts for root culture were made by Robbins and Kotte during 1920s. Later on, many workers tried for achieving successful root cultures. In 1934, it was White who successfully cultured the continuously growing tomato root tips.
Subsequently, root culturing of a number of plant species of angiosperms as well as gymnosperms has been done successfully. Root cultures are usually not helpful for giving rise to complete plants but they have importance’s of their own. They provide beneficial information regarding the nutritional needs, physiological activities, nodulations, infections by different pathogenic bacteria or other microbes, etc.
4. Shoot Culture:
Shoot cultures have great applicability in the fields of horticulture, agriculture and forestry. The practical application of this method was proposed by Morel and Martin (1952) after they successfully recovered the complete Dahalia plant from shoot-tips cultures.
Later on, Morel realized that the technique of shoot culturing can prove to be a potent method for rapid propagation of plants (i.e. Micro propagation). In this technique, the shoot apical meristem is cultured on a suitable nutrient medium. This is also referred to as Meristem Culture (Fig. 8).
The apical meristem of a shoot is the portion which is lying beyond the youngest leaf primordium. Meristem tip culture is also beneficial for recovery of pathogen-free specially virus-free plants through the tissue culture techniques. Various stages in this culture process are the initiation of culture, shoot multiplication, rooting of shoots and finally the transfer of plantlets to the pots or fields.
5. Protoplast Culture:
A protoplast is described as a plasma membrane bound vesicle which consists of a naked cell formed as a result of removal of cell wall. The cell wall can be removed by mechanical or enzymatic methods. In-vitro culturing of protoplasts has immense applications in the field of plant biotechnology.
It not only serves for genetic manipulations in plants but also for biochemical and metabolic studies in plants. For protoplast culture, firstly the protoplasts are isolated from the plants utilizing some chemical or enzymatic procedure.
At present, there are available a number of enzymes which have enabled the isolation of protoplasts from almost every plant tissue. After isolation of protoplasts, they are purified and then tested for their viability. Finally the purified viable protoplasts are cultured in-vitro using suitable nutrient medium which is usually either a liquid medium or a semisolid agar medium.
6. Haploid Production:
Haploid plants are those which contain half the number of chromosomes (denoted by n). Haploids can be exploited for benefits in the studies related to experimental embryogenesis, cytogenetics and plant breeding. Haploids have great significance in field of plant breeding and genetics. They are most useful as the source of homozygous lines.
In addition, the in-vitro production of haploids also aids for induction of genetic variabilities, disease resistance, salt tolerance, insect resistance, etc. Presently, attention is being focused on improving the frequencies of haploid production in their advantageous utilization for economic plant improvement.
There are two approaches for in-vitro haploid production. These are:
(a) Androgenesis:
The technique of production of haploids through anther or microspore culture is termed as androgenesis. It is a method par excellence for the large scale production of haploids through tissue culture.
Androgenesis technique for haploid production is based on the in-vitro culture of male gametophyte i.e., microspore of a plant resulting into the production of complete plant from it. It is achieved either by another culture or by microspore (pollen) culture.
The technique of another culture is quicker for practical purposes and is an efficient method for haploid production.
But sometimes during another culture, the plantlets may originate from different other parts of anther also (along with from the pollens). On the other hand, microspore culture is free from any uncontrolled effects of the anther wall or other tissues. Microspore culture is ideal method for studying the mutagenic and transformation patterns (Fig. 9).
(b) Gynogenesis:
It is an alternative source of in-vitro haploid production. It refers to the production of haploid plant from ovary culture or ovule culture. The method of gynogenesis for haploid production has been successful, so far, in a very few plants only, hence it is not a very popular method for in-vitro production of haploids. Thus, androgenesis is preferred over gynogenesis.
7. Embryo Culture:
The technique of embryo culture involves the isolation and growth of an embryo under in-vitro conditions to obtain a complete viable plant. First success for embryo culture was made by Hannig in 1904 when he isolated and cultured embryos of two crucifers namely Cochleria and Raphanus. Embryo culture is used widely in the fields of agriculture, horticulture and forestry for production of hybrid plants.
This technique allows the detailed study about the nutritional requirements of embryos during different developmental stages. Also, it helps for identifying the regeneration potential of embryos. Embryo culture is advantageous for in-vitro micro propagation of plants, overcoming seed dormancy and for production of beneficial haploid plants.
8. Endosperm Culture (Triploid Production):
Endosperm tissue is triploid therefore the plantlets originating by the culture of endosperm are also triploid.
In majority of flowering plant families (exceptions being Orchidaceae, Podostemaceae, Trapaceae which lack endosperm) the endosperm tissues are present. Endosperm is formed after the double fertilization of one male nucleus with two polar nuclei. Immature endosperm has more potential of growth in culture especially among the cereals.
Endosperm culture has provided a novel strategy for plant breeding and horticulture for the production of triploid plantlets. It is an easy method for production of a large number of triploids in one step.
Moreover, it is much more convenient that the conventional techniques like chromosome doubling by crossing tetraploids with diploids for triploid induction. Full triploid plants of endosperm origin have been produced in a number of plant species like Populus, Oryza sativa, Emblica officinalis, Pyrus malus, Prunus, etc.
The triploid plants are usually seedless therefore this technique is most beneficial for increasing the commercial value of fruits like apple, mango, grapes, watermelon, etc. In addition to all the above described applications, endosperm culture is helpful for studying biosynthesis and metabolism of certain natural products also.
Essay # 9. Applications of Plant Tissue Culture:
1. Germplasm conservation mainly in the form of cryopreservation of somatic embryos or shoot apices, etc.
2. Large scale production of useful compounds and secondary metabolites by using genetically engineered plant tissue cultures.
3. Technique of micro propagation for enhancing the rate of multiplication of economically important plants.
4. Eradication of systemic diseases in plants and raising disease free plants.
5. Soma-clonal variations are useful sources of introduction of valuable genetic variations in plants.
6. Helps plants in imparting resistance to antibiotics, drought, salinity, diseases, etc.
7. Somatic hybrids and cybrids overcome species barriers and sexual incompatibility and produce hybrid plants with desired combination of traits.
8. Embryo culture helps in overcoming seed sterility and dormancy.
9. Haploid production in culture helps to solve various problems of genetic studies and thus aids the plant breeders for producing new varieties.
10. Production of synthetic seeds via somatic embryo differentiation for commercially important plants helps to achieve increased agricultural production.
11. Large scale production of biomass energy.
12. Plant tissue culture aids in producing the genetically transformed plants.
13. Early flowering can be induced by in-vitro culturing of plants so as to attain commercial benefits.
14. Triploids as well as polyploid plants can also be produced by tissue culture techniques for uses in plant breeding, horticulture and forestry.
15. Seedless fruits and vegetables can be produced by following the endosperm culture method which add to their commercial values.
16. Increased Nitrogen fixation ability can be achieved through association of tissue culture techniques with genetic engineering.
17. Callus cultures are useful in plant pathology as they act as an effective tool in the study of mechanism of disease resistance and susceptibility.
18. Different tissue culture techniques help us to study various biosynthetic processes, physiological changes and cytogenetic changes.
Essay # 10. Morphogenesis:
Literal meaning of the term morphogenesis is origin of form. It includes all the activities which are involved in the formation of a complete individual starting from the cells/tissues in culture. Various internal as well as external factors affect the morphogenic potential of the cells.
The study of such factors and their effect on the regeneration of cells constitutes the morphogenesis. The appearance of the plants may be altered by the changes in growth environment. The causal factors of such changes may be studied properly by morphogenesis.
Morphogensis may also be termed as developmental morphology and it may be defined as “the branch of biology that deals with the causes and activities during the organization of a complete individual plant.” Another definition of morphogenesis can be given as the study of various factors which affect the organic form and describe the growth patterns of the individual.
All those anatomical and physiological events which are involved in the growth and development of an organism are included in the morphogenetic studies.
Differences in the morphogenetic phenomena lead to the differences in the form of characteristic organs or structures. In simple words, morphogenesis may be described as the developmental pathways in differentiation as a result of which the recognizable tissues are formed.
Cell culturing has blossomed into such a technology which is capable of solving a number of problems with an impact on morphogenesis of plants. This is so because, through the elegant system of plant cell/tissue culture, it is possible to have an effective control of exogenous factors which affect the differentiation starting with the callus or a single cell or tissue.
Factors of Morphogenesis:
There are many such factors which influence the morphogenic potential of cells by affecting important developmental activities. For e.g.,
i. Several experiments have shown that certain growth factors (like cytokinin) induce the somatic embryos in culture by changing the cell polarity.
ii. Some chemical factors promote asymmetric divisions in the cultured cells thus resulting in difference in form.
iii. Different concentrations of 2, 4-D, auxins, etc. alter the development of organ in culture by causing the altered cell elongation or rate of cell division.
iv. With the increase in culture duration, the organogenic differentiation shows decline. All such examples show that different factors have different type of effect on different developmental activities. Such dynamic and causal aspects of organic forms are studied in morphogenesis.
A list of the factors having great importance in deciding the morphogenic pattern is given below:
i. Physical factors like light, temperature, pH.
ii. Chemical factors like C/N ratio, oxygen content, carbohydrates (sugar) concentrations, etc.
iii. Growth hormones like Auxin, Cytokinin, Gibberellins, ABA, etc.
iv. Water
v. Culture duration
vi. Mechanical strains, pressures, bending, etc., may cause changes in plane of cell division, thus may cause difference in morphogenic potential.
vii. The regenerability of explant may be affected by factors like the organ from which it is taken, the physiological state of the explant, its size, etc.
viii. Electrical stimulation.
ix. Changes in gene expression in cells in culture.
x. Genotype and status of nucleic acids in different cells.
xi. Repeated subculture of a totipotent callus may also result in reduction or complete loss of morphogenetic potential.
Role of Morphogenesis:
As described above, morphogenesis includes all those events which occur during the growth and development of an organism. Thus, it is very important for providing a satisfactory explanation of various biological growth patterns. It also aims on studying and correlating different environmental factors with changes in the morphogenetic patterns and/or potential.
Major contributions for morphogenetic studies in India have been provided by Department of Botany, University of Delhi. Numerous experiments on a wide variety of subjects were performed by the department to study the morphogenesis by utilizing tissue culture techniques.
De-novo organ initiation and formation under aseptic culture form a fascinating subject to study the effect of different factors on the morphogenetic patterns.
Different objectives of morphogenesis are summarized below:
i. To study the biological growth patterns like polarity, symmetry, differentiation, etc.
ii. To provide an explanation to all such growth patterns.
iii. To relate morphogenesis with other major biological sub sciences.
iv. Advantageous utilization of tissue culture techniques to study morphogenetic patterns.
v. Determining interacting influence of growth hormones in shoots or root regeneration.
vi. To study the effects of repeated sub-culturing on the regeneration potential of a totipotent callus.
vii. To analyse the growth regulation in differentiation of plant species by trial and error.
viii. Study of effect of osmotic inhibition using high levels of sucrose during shoot formation.
ix. Study of role of light and temperature in flowering.
x. Exploring the correlation between nuclear state and potential for differentiation and regeneration.
Essay # 11. Subculture or Secondary Cell Culture:
The process of transferring the cultured cells in a fresh nutrient medium is called as sub-culturing, and the cell cultures which are sub-cultured (i.e., inoculated in a separate medium) are called as subculture or secondary cell cultures or secondary cell lines.
It is important to subculture the organ and tissues to fresh medium to avoid the condition of nutrition depletion and drying of medium. It is possible to maintain the plant cell and tissue cultures for indefinitely long time durations if they are regularly subculture in a serial manner (Fig. 10).
Essay # 12. Soma Clonal Variation:
Soma clonal variations may be defined as those variations which occur in the cultured cells/tissues or plants regenerated from such cells in-vitro. Soma clonal variations are usually heritable for qualitative as well as quantitative characters of plants. Soma clonal variants have proved as an alternate tool to plant breeding for production of improved varieties of plants.
Gene mutations and changes in the structure, number of chromosomes are the main causes of production of soma clonal variants. A number of new varieties of cereals, oil seeds, fruits, tomatoes, etc., possessing disease resistance, better quality, better yield etc., have been generated through soma clonal variations. Some of those crop species are potato, tomato, oats, wheat, rice, maize, datura, carrot, soybean, etc.
In simple words, the variability which is generated by the use of a tissue culture cycle is termed as soma clonal variation. Soma clones is a general term which is used to describe those plants which have been derived from any type of somatic cell cultures.
Essay # 13. Somatic Hybrids and Cybrids:
Somatic hybridization may be described as the production of hybrid cells by the fusion of protoplasts of somatic cells derived from two different plant species/varieties. It is immensely helpful for generating new and improved hybrid varieties of plant that may have characters of a completely different species.
For example, ‘Pomato’ is a somatic hybrid which is produced by the fusion of protoplast of somatic cells from potato and tomato which are totally different species. A cybrid is a cytoplasmically hybrid cell which has the cytoplasm of both fusing cells but nucleus of only one fusing cell. The process of production of a cybrid is called cybridisation.
Steps involved in somatic hybridization/cybridization (Fig. II) are given below:
a. Protoplast isolation from parent plant using any mechanical or enzymatic method.
b. Fusion of isolated protoplasts derived from two different parents either by utilizing chemical fusogens (like NaNO3, Polyethylene Glycol or high pH-high Ca++ treatment) or by the electro-fusion method.
c. Selection of the hybrid cells is done after fusion process.
d. The selected somatic hybrids/cybrids are then verified for hybridity. This is done to check whether the hybrid is carrying the desired characteristics of both parents or not.
e. On successful verification and characterization, the somatic hybrids or cybrids are cultured for regenerating into the plantlets with desired characters.
Somatic hybridization overcomes the sexual incompatibility barriers and it enables to produce interspecific as well as inter-generic crosses in plants. It helps in imparting disease-resistances and improving the quality characters in plants. It is an immensely beneficial tool for study of cytoplasmic genes and their expressions.
Essay # 14. Micro-Propagation:
Tissue culture helps in the rapid propagation of plants by the technique of micro-propagation or clonal propagation in-vitro. The asexually produced progeny of a cell or individual is called as clone and the clones have an identical genotype.
Micro propagation is the technique of in-vitro production of the clones of plants i.e., it produces the progeny plants which have an identical genotype as their parents, by cell, tissue or organ culture (Fig. 12). It helps in the production of plants in large number starting from a single individual. It serves as an alternate method to conventional vegetative propagation methods.
Micro propagation may be achieved by shoot tips, axillary buds, adventitious buds, bulbs or somatic embryos. A number of plant species are being clonally propagated in vitro, specially the commercially valuable plants like orchids and forest trees.
Some of the important plants that have been micro propagated on large scales are:
Orchids like Cymbidium, Dendrobium, Aranda, Vanda, Odontoma, Vanilla, etc.
Forest trees like Tectona grandis, Biota, Cedrus deodara, Eucalyptus, Picea, Pinus, etc.
The technique of micro propagation generally involves four stages. Each of these stage has its own requirements.
The stages in general technique of micro-propagation are described below:
Stage I. Initiation:
This stage also involves the preparatory process for achieving better establishment of aseptic cultures of explant. Suitable explant is selected from the mother plant. Then, the explant is sterilized and transferred to the nutrient medium for culture.
State II. Multiplication:
This is the most important stage of micro propagation. In this stage, there occurs the proliferation or multiplication of shoots (or embryoids) from the explant on medium. It occurs either by the formation of an intermediary callus or by induction of adventitious buds directly from the explant.
Stage III. Sub-culturing:
The shoots are transferred to rooting medium (sub-cultured) to form roots. As a result, complete plantlets are obtained.
Stage IV. Transplantation:
In this stage, the regenerated plantlets are transferred out of culture. These are grown in pots followed by field trials.
Advantages of Micro Propagation:
1. Rapid multiplication of disease free plants.
2. Rapid multiplication of commercially important plants.
3. Maintenance of genetic uniformity.
4. Technique does not depend on seasons and is capable of producing plants all round the year.
5. Technique is valuable in cases where only limited explant is available.
Limitations of Micro Propagation:
1. The technique is costly.
2. It requires proper skill.
3. Many tree crops, including gymnosperms, cannot be multiplied by clonal propagation.
4. Clonal propagation in some cases may lead to the formation of off-types rather than clones, after many generations.
5. If culture is contaminated, then the pathogen gets multiplied to very high levels and becomes difficult to handle.
Essay # 15. Artificial Seed:
These are also called as synthetic seeds. These are living seed like structures which are capable of giving rise to plants when sown in the field. An artificial seed is made of a somatic embryo (S.E.) encapsulated with a protective layer of a gel which protects it from desiccation or microbial attack (Fig. 13).
Essay # 16. Cryopreservation:
It means preservation at ultralow temperature:
This technique is used mainly for long term storage of germplasm and thus helps in conservation of nature also. Plant tissues and organs are cryopreserved usually in liquid Nitrogen which has a temperature of 196°C. Cryopreservation technique has proved to be one of the most reliable methods for long term storage and preservation of plant germplasm in the form of pollens, shoot-tips, embryos, callus, protoplasts, etc.
Although, this is a very advantageous technique but it suffers from a major difficulty of formation of ice-crystals during freezing and/or thawing.
These ice-crystals may cause damage to the preserved material. To prevent the formation of ice-crystals during cryopreservation, some special chemicals are used which are called as cryoprotectants. A few common cryoprotectants are glycol, sucrose, proline, Dimethyl Sulfoxide (DMSO), Polyethyleneglycol (PEG), etc..