ADVERTISEMENTS:
Here is an essays on ‘Lipids’ for class 8, 9, 10, 11 and 12. Find paragraphs, long and short essays on ‘Lipids’ especially written for school and college students.
Essay on Lipids
Essay Contents:
ADVERTISEMENTS:
- Essay on the Introduction to Lipids
- Essay on the Functions of Lipids
- Essay on the Structure of Lipids
- Essay on the Classification of Lipids
- Essay on the Properties of Lipids
Essay # 1. Introduction to Lipids:
Lipids are defined to be the esters of higher aliphatic acids with a characteristic property of insolubility in water and solubility in fat solvents like chloroform, ether, benzene and carbon tetrachloride. Due to the presence of replaceable OH group, alcohols behave like alkalies and as such, combine with acids, forming salts and H2O. These salts of alcohols with acids are technically known as esters.
ADVERTISEMENTS:
They may be represented as (HOOC—F represents fatty acids):
Fatty acid esters may be formed with alcohols other than glycerol, such as waxes. The commonest types of fatty acids occurring in natural fats usually contain even number of carbon atoms and are straight- chain derivatives. They may be saturated or unsaturated. Saturated fatty acids do not contain double bonds, but unsaturated fatty acids contain one or more double bonds or ethylene groups. The general formula of fatty acids is CnH2n+1COOH.
The common saturated fatty acids are:
(a) Butyric acid (C3H7COOH),
(b) Palmitic acid (CH3(CH2)14COOH), and
(c) Stearic acid (CH3(CH2)16COOH).
The unsaturated fatty acids are:
(a) Oleic acid (CH3(CH2)7. CH=CH(CH2)7 COOH) with one double bond,
ADVERTISEMENTS:
(b) Crotonic acid (CH3 – CHCH-COOH),
(c) Linoleic acid (C17H31COOH) containing two double bonds, and
(d) Linolenic acid (C17H29COOH) containing three double bonds.
Consequently the oleic acid is unsaturated, and is liquid at ordinary temperature. Certain other fatty acids may be present in small amounts. Formerly it was believed that natural fat contains fatty acids with an even number of carbon atoms. Lately, thin- layer and gas chromatographic techniques have revealed the presence of a good number of fatty acids containing odd carbon atoms also.
ADVERTISEMENTS:
The simple triglycerides are those in which glycerol remains united with three molecules of the same type (identical) fatty acids. The naturally occurring fats are generally all of mixed type, in which glycerol remains combined with molecules of different types of fatty acids.
Essay # 2. Functions of Lipids:
i. Food Material:
Lipid provides a food with a high calorific value (1 gm produces about 9.3 kilocalories of heat).
ADVERTISEMENTS:
ii. Food Reserve:
Lipid acts as reserved food materials, because it is readily stored in the body on account of its insolubility in aqueous solutions.
iii. Heat Insulations:
Being deposited in subcutaneous tissue lipid acts as an insulator. Subcutaneous fat contents are higher in aquatic animal (i.e., whale) and in animals living in cold climates.
ADVERTISEMENTS:
iv. Solvents:
Lipid acts as vehicle of natural fat-soluble vitamins—A, D and E.
v. Structural Constituent:
Lipid is a structural component of the cell membrane.
vi. Fat Transport:
Phospholipids play an important role in absorption and transportation of fatty acids.
ADVERTISEMENTS:
vii. Hormone Synthesis:
Adrenocorticoids, sex hormones, vitamin D and cholic acids are synthesised from cholesterol.
Essay # 3. Structure of Lipids:
Insulators and Cushions:
In general, fat stored in fat cells can be mobilised for energy when caloric intake is less than caloric expenditures. Some types of fat, however, seem to be protected from metabolic activity. Large masses of fatty tissue, for example, surround mammalian kidneys and serve to protect these precious organs from physical shock.
For reasons that are not understood, these fat deposits remain intact even at times of starvation. Another mammalian characteristic is a layer of fat under the skin, which serves as thermal insulation. This layer is particularly well developed in sea-going mammals.
ADVERTISEMENTS:
Among humans, females characteristically have a thicker layer of sub-dermal (“under-the-skin”) fat than males. This capacity to store fat, although not much admired in our present culture, was undoubtedly very valuable 10,000 or more years ago.
At that time, there was no other reserve food supply, and this extra fat not only nourished the woman but more important, the unborn child and the nursing infant, whose ability to fast without damage is much less than that of the adult. Thus many of us are strenuously dieting off what millennia of evolution have given us the capacity to accumulate.
Lipids and Membranes:
Lipids also play extremely important structural roles. The lipids most important for structural purposes are phospholipids. Like fats, the phospholipids are composed of fatty acid chains attached to a glycerol backbone. In the phospholipids, however, the third carbon of the glycerol molecule is occupied not by a fatty acid but by a phosphate group to which another polar group is usually attached.
Phosphate groups are negatively charged. As a result, the phosphate end of the molecule is hydrophilic and the fatty acid portions are not. The consequences are shown in Figure 3.12. According to the present model, this arrangement of phospholipid molecules, with their hydrophilic heads extended and their hydrophobic tails clustered together, forms the structural basis of the cell membrane.
Waxes:
Waxes are also a form of structural lipid. They form protective coatings on skin, fur, feathers, on the leaves and fruits of higher plants, and on the exoskeletons of many insects.
Essay # 4. Classification of Lipids:
Lipids are classified as:
(a) Simple lipids;
(b) Compound lipids;
(c) Sterols or steroids;
(d) Hydrocarbons.
I. Simple Lipids:
True Fats:
These are esters of fatty acids with glycerol (CH2OH-CHOH-CH2OH) and are generally called triglycerides. On hydrolysis with water at high temperature and pressure in an autoclave in the presence of acids, triglyceride may yield three molecules of fatty acids and one molecule of glycerol.
Furthermore, if the triglycerides are boiled with alkali, such as NaOH or KOH then glycerol and Na or K salt of fatty acids (soap) are formed. Triglycerides having unsaturated fatty acids may be hydrogenated at their double bonds in the presence of nickel catalyst. Commercial cooking fats are mostly hydrogenated fats and become hard due to hydrogenation.
Waxes:
These are esters of fatty acids with alcohol other than glycerol. In the human body the commonest waxes are the cholesterol esters. They are most abundant in the blood, suprarenal glands, the gonads and the sebaceous glands of the skin. Like true fats, the natural waxes are also complex mixtures. They are solid at ordinary temperature and are not as readily hydrolysed as fats.
The three common waxes are as follows:
(a) Beeswax:
It is myricyl (C30H61OH) palmitate.
(b) Lanoline:
It is a mixture of cholesterol palmitate, stearate and oleate.
(c) Spermaceli: (in the skull of sperm whale):
It is acetyl palmitate.
II. Compound Lipids:
Fats combined with other non-fatty prosthetic groups.
Phospholipids or Phosphatids:
Fats containing phosphoric acid and a nitrogenous base in the molecule.
So far three types of phosphatids have been identified:
(a) Lecithin:
It is composed of 1 molecule of glycerol, 2 molecules of fatty acids and 1 molecule of phosphoric acid. Another nitrogenous base (choline) remains attached to the phosphoric acid. When placed in water it swells up and readily forms an emulsion. Due to presence of acid and basic groups, it combines both with bases and acids. It is a pale-yellow solid which readily absorbs water and oxygen when exposed to air and light, and is thus transformed into a dark-plastic mass.
(b) Kephalin (Cephalin):
It is similar to lecithin except that instead of choline the nitrogenous base here, is ethanolamine. It occurs in two forms, α-kephalin and β-kephalin. Kephalin and lecithin occur together in the tissue and their properties are generally similar. Howell claims that the substance thrombokinase which initiates blood clotting is identical with kephalin. It is found especially in nerve tissues, egg-yolk, milk, etc.
(c) Sphingomyelin:
Its constituents are same as lecithin except that glycerol is replaced by another unsaturated nitrogenous alcohol called sphingosine. It is stable in light and air, and is a white crystalline solid. It is also found in the animal tissues especially in nerve tissues, egg-yolk, milk, etc. Due to the presence of two asymmetric carbon atoms, 4 stereo-isomers are possible. The natural forms are dextrorotatory.
(d) Plasmalogen:
It is another type phospholipid present in the brain, liver and muscle tissue. After hydrolysis it yields cholamine esters of α and β-glycerophosphoric acid along with a mixture of higher aldehyde. Both palmitic and stearic aldehydes have been found to be the constituents of plasmalogen.
Glycolipids (Cerebrosides):
In general properties they resemble the sphingomyelins. It is also named as glycosphingosides. They are especially abundant in the brain. They are characterised by the presence of galactose in the molecule. For this reason they are also called galactolipids. Three cerebrosides have been identified, e.g., phrenosin, kerasin and nervon. The existence of another member of this class oxynervon has also been suggested.
They all contain fatty acids, galactose and nitrogenous base – sphingosine. The difference between cerebrosides is due to the difference in fatty acids. The function of cerebrosides is not known as yet. Largest amount of them have been found in brain white matter and myelin sheath. In Gaucher’s disease a large amount of cerebrosides have been found to occur in liver and spleen.
Gangliosides:
They contain sphingosine, fatty acid, hexose, hexose-amine and sialic acid. Gangliosides have been found in nerve cells and also in spleen and red blood cells. In Niemann-Pick disease a large amount of them are present in brain.
Lipoproteins:
Protein molecules conjugated with neutral fats (triglycerides), cholesterol or phospholipids, e.g. α and β-lipoprotein in plasma. Changes in one or other fraction have been noted-in coronary disease, in acidosis accompanying diabetes, in essential hyperlipaemia, etc. Lipoprotein is also present in cell membrane, milk, egg-yolk, etc.
Sulpholipids:
They are sulphuric ester of sphingosine cerebronic acid and galactose (cerebron). Largest amount has been observed in white matter of brain and to a lesser extent in liver, kidney, salivary gland, testis, etc.
III. Steroids or Sterols:
Chemistry:
Steroids or sterols are not true fats. Steroids are derivatives of phenanthrene, and have the parent nucleus (perhydrocyclo pentanophenanthrene) with 17 carbon atoms consisting in 3 hexagon and 1 pentagon rings and hydrogen atoms are added until the valency reaches 4. When the natural steroids have a methyl group at C-13 and generally at C-10 and possess one or more alcoholic groups, then these secondary alcohols are sterols.
Steroids are found free or in ester bonds with fatty acids (cholesterides) and can be extracted by organic solvents along with fats. Cholesterol, coprosterol, ergosterol, male and female sex hormones, adrenocortical hormones and vitamin D are major steroids.
Sterols cannot be hydrolysed by NaOH. Hence, they are not saponifiable. Otherwise, their physical and chemical properties are very similar to those of fats. With fatty acids they form waxes. The various members of this group can be separated by fractional distillation.
Distribution:
Sterols are widely distributed in both animal and vegetable kingdoms. In the plants they exist chiefly as phytosterols. In the animals they are present in all cells, both in the cell membrane as well as in the cytoplasm. They always remain along with phospholipids. They are found both as free sterols as well as sterides. Certain tissues are very rich in this substance, such as the nervous tissue, suprarenal cortex, gonads, etc.
Synthesis, Destruction and Excretion:
Sterols can be synthesised in the body from non-sterol sources. It has been definitely proved in the case of cholesterol. The body can destroy sterols to some extent but the power is very limited. Large quantities are excreted through the skin in sebum formed by the sebaceous glands, in the stool as coporsterol and in the urine as the sex hormones and traces of cholesterol.
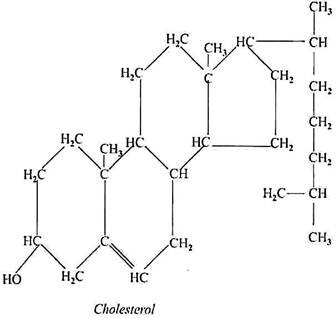
Cholesterol:
Chemistry and Properties:
It is complex monohydric secondary alcohol being a very important member of the sterol class. With fatty acids it forms waxes. It is a stable white crystalline substance, insoluble in water but readily soluble in chloroform, ether, alcohol and other fat solvents. The crystals have a rhombic or rectangular shape, with one corner; broken off.
Cholesterol when mixed with fats or oils helps them to absorb a large volume of water due to formation of water in oil emulsion. This sterol is present in high amount in nervous tissue and being a poor conductor of electricity, serves the purpose of an insulating cover over the structures through which nerve impulses, which are electrical in nature, are conveyed.
Distribution:
The broad facts about the distribution of cholesterol are given below:
(a) It is present in all cells- both in the cell membrane and cytoplasm,
(b) All body fluids contain cholesterol excepting cerebrospinal fluid, in which the amount is negligible,
(c) It may exist in the Free State as well as in the form of esters, but these two forms are not equally distributed everywhere. In bile it is present only in the free form,
(d) Brain (17%) and suprarenals have the richest supply. In the former it is found mainly in the free form,
(e) Blood cholesterol. Normal blood cholesterol varies between 0.15% to 0.2%. The total quantity is equally distributed between plasma and corpuscles. But in the corpuscles it is present chiefly in the free form, while in the plasma the major part remains as esters,
(f) The free cholesterol content of a tissue is characteristic and normally remains very constant but the cholesterol esters may vary in amount,
(g) The distribution of cholesterol in different types of muscles indirectly proportional to their degree of activity. The cardiac and smooth muscles have richer cholesterol content than the voluntary muscles. Moreover, the amount of cholesterol in any tissue is roughly proportional to their degree of activity, and
(h) Cholesterol and phospholipids always remain together. Moreover, there appears to be a definite ratio between phospholipids and cholesterol for each tissue. So that they not only remain together but also in a definite proportion.
Interrelation with other Sterols:
Cholesterol is very closely related to many other sterols of immense physiological importance, for instance:
(a) The active principles of adrenal cortex,
(b) The male sex hormones,
(c) The female sex hormones,
(d) Vitamin D and ergosterol, and
(e) Cholic acid, etc.
Excepting in the case of cholic acid, it has not been proved that cholesterol acts as the mother substance for the other sterols. But since, in the laboratory the male and female sex hormones have been synthesised from cholesterol, it is possible that it may serve as the precursor from which most, if not all, of the physiologically important sterol compounds are derived. For further details vide ‘Cholesterol Metabolism’.
IV. Hydrocarbons:
Hydrocarbons are the substances which have got no structural relationship to fatty acids but yet they are grouped as lipids only because of their similar solubility properties. These are squalene, carotenes, vitamins A, E and K.
Essay # 5. Properties of Lipids:
Physical:
i. Solubility:
They are insoluble in water but soluble in ether, chloroform, benzene, petroleum-ether, carbon tetrachloride, hot alcohol, etc. (True fats are colourless, odourless and tasteless)
ii. Consistency:
At ordinary temperature, some fats remain solid whereas others in liquid form. Unsaturated fats are generally liquid. Those fats which remain liquid at ordinary temperature are called oils.
iii. Melting Point:
Different fats have different melting points. For instance, pure tristearin melts at 71.5°C.; beef fat melts at 49.5°C.; human fat at 17°C. Glycerides of unsaturated fatty acids have lower melting points than those of saturated fatty acids. It is interesting to note that fats from a particular species of animal have got somewhat characteristic melting point.
iv. Specific Gravity:
It is less than 1.0, i.e., lighter than water. Solid fats are lighter than the liquid fats.
v. Spreading and Surface Tension:
When liquid fat is poured on water, it spreads uniformly over the surface in a unimolecular layer and reduces the surface tension of water.
Chemical:
i. Reaction:
True fat is neutral in reaction and colourless, but after exposure to air it becomes acid in reaction and yellow in colour owing to partial hydrolysis of fats and oxidation of the unsaturated fatty acids. When thus altered, the fat is said to become rancid. Antioxidants’ present in natural fats prevent this rancidity to some extent.
ii. Hydrolysis:
By boiling with acids or alkalies or by applying superheated steam, fats can be completely hydrolysed into fatty acids and glycerol.
iii. Saponification number (Value):
When fats are boiled with alkali, soaps are formed and the process is termed saponification. In this way Na, K, Ca, Mg, Li soaps are formed by boiling fats with corresponding hydroxides. By the term saponification number (saponification value) of a fat is meant the amount of potassium hydroxide (KOH) in mg required to saponify completely 1 gm of fat. This value gives the idea about the average molecular size of the fatty acids present in the glycerides of the fat and varies inversely with the molecular weight of the fatty acids.
ADVERTISEMENTS:
iv. Addition Reactions:
Fats containing unsaturated fatty acids can take up other elements and thus become saturated.
Two such addition reactions are of physiological interest:
(a) Iodine Number:
Under suitable conditions double bonds of unsaturated fatty acids may be made to take up halogens, bromine or iodine. By the term iodine number (regardless of halogens used) of a fat, is meant the number of grams of iodine, taken up by 100 grams of that particular fat. The degree of unsaturation of a particular fat can be easily assessed by determining its iodine number. Fats derived from a particular source have generally got a characteristic iodine number.
(b) Hydrogenation:
Just like iodine, an unsaturated fat may be made to take up hydrogen atoms in the presence of a catalyst, especially nickel. For instance, addition of two H atoms to oleic acid would give the corresponding saturated acid, the stearic acid. In this way edible fats can be made from cheaper unsaturated oils. The so-called vegetable ghee is manufactured by this process.
v. Oxidation:
Unsaturated fatty acids when exposed to air are easily oxidised to form various aldehydes and ketones, which may react further and form complex resinous compounds. For this reason, linseed oil when spread in a thin layer, is rapidly converted into a tough waterproof film which adheres closely upon the surface. Due to this property such oils are called drying oils. This property is made use of in the manufacture of paints, varnishes, linoleum, etc.
vi. Acetyl Number:
It is defined as the mgs of KOH required to combine with the acetic acid liberated by the saponification of 1 gm of acetylated fat.
vii. Rancidity:
It is a chemical change that may result unpleasant odour and taste within the natural fat due to keeping for a long time. This is due to hydrolysis of fat into free fatty acids and glycerol or mono and di- glycerides. Acid number determines the rancidity or free fatty acids present in the fat. It is defined as mgs of KOH required to neutralise the free fatty acids present in 1 gm of fat. Light hastens and either natural ‘antioxidants’ or added reducing agents retard the process.
viii. Reichert-Meissl number or Volatile acid Value:
It is ml of 0.1 normal (N) KOH required to neutralise volatile fatty acids obtained during distillation of 5 gm of hydrolised fat after it has been saponified with KOH and then made acid with H3PO4 or H2SO4. This value actually determines the presence of total fatty acids of low molecular weight.