ADVERTISEMENTS:
In this essay we will discuss about the life cycle of Pinus, explained with the help of suitable diagrams.
Sporophyte of Pinus:
Pinus is a tall evergreen tree giving rise to a series of widespread horizontal branches (Fig. 1.57A). In each year, a whorl of branches is produced in the axil of scale leaves. The branching is restricted to the upper part of the stem, thus giving the tree a pyramid-like appearance. The main stem is cylindrical and covered with scaly bark.
The branches are dimorphic, bearing two types of shoots: long shoots and dwarf shoots, or spurs or brachyblasts (Fig. 1.57B). Pinus exhibits two types of leaves, the scale leaves and the green acicular foliage leaves called needles (Fig. 1.57B). The plant has a tap root system which becomes elongated at maturity and possesses strong lateral roots.
The plants are monoecious where the male and female cones are borne on separate branches in the same plant. 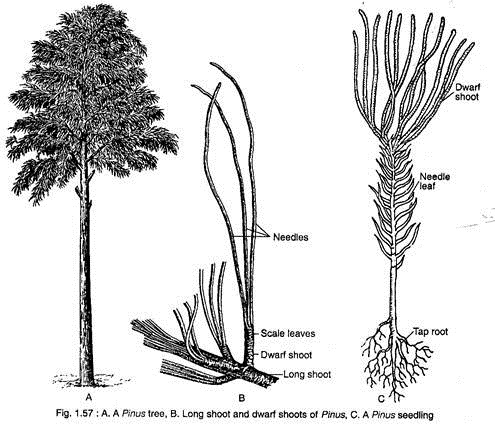 Reproduction:
Reproduction:
Pinus reproduces sexually. Pinus is monoecious, but the male and the female cones are produced on separate branches of the same plant. The male cones develop on the lower branches, while the female cones are formed on the upper branches. The male cones, which replace the dwarf shoots, develop in clusters on the base of the current year’s long shoot at early spring (Fig. 1.61 A).
The number of male cones in a cluster varies considerably from 15 (P. wallichiana) to 140 (P. roxburghii). At the onset of spring, the male cones fall off and simultaneously the young female cones are borne in pairs or in clusters round the tip of the long shoot (Fig. 1.61 B).
The female cones grow very slowly and the growth may persist for several years. Thus, the female cones of different ages may be seen in acropetal succession in the long shoot (Fig. 1.61B).
i. Male Cone:
ADVERTISEMENTS:
The male cone is small (2-4 cm in length) and oval in shape that develops in the axil of scale leaves. The male cone has a central axis on which 60-150 microsporophylls are spirally arranged around the axis (Fig. 1.62A).
A single microsporophyll is a membranous stalked structure with a distal expanded roughly triangular sterile part called apophysis (Fig. 1.62B). Each microsporophyll bears two sac-like microsporangia on the abaxial surface.
The development of microsporangia is of eusporangiate type, i.e., it develops from a group of hypodermal cells of the microsporophyll. A mature microsporangium consists of a multilayered wall, tapetum and microspore mother cells (Fig. 1.62D). Each microspore mother cell — by meiotic division — produces four microspores or pollen grains.
Thus, at maturity, a single microsporangium contains numerous pale yellow pollen grains. The pollen grains are boat-shaped with monosulcate apertures and are bounded by two concentric wall layers: the outer thick exine and the inner thin intine (Fig. 1.62C).
The exine on the lateral sides of the pollen is expanded to form two wings (sacci). Thus the pollen grains of Pinus are bisaccate indicating their anemophilous mode of pollination. The dehiscence of sporangia takes place by longitudinal slit in dry and warm environment.
Pinus is wind-pollinated (anemophilous). The pale-yellow pollen gains are released into the air in a large quantity, so that a pine forest appears yellow at the time of pollination. This is popularly called ‘sulphur showers’ which occurs specially in the spring when pine trees are shaken by strong winds.
ii. Female Cone:
ADVERTISEMENTS:
Female cones are produced in pairs or in clusters in the axil of the scale leaves. The female cones mature very slowly. The first year young cone (Fig. 1.61 A, B) is small (1-2 cm in length), soft, compact and red-green in colour. The second year cone (Fig. 1.61 A, B) is comparatively large (5-8 cm in length), woody, compact and green in colour.
The fully matured third year cone is much larger (15-60 cm in length), woody, loose and brown in colour. Here megasporophylls are separated from each other due to the elongation of the cone axis.
The female cone of Pinus represents a compound shoot; it is a complicated structure. The female cone is composed of a central axis on which 80-90 megasporophylls, axillary to bract scale/scale leaves, are arrange spirally (Fig. 1.63A).
The bract scale and ovuliferous scale thus form a seed-scale complex.
ADVERTISEMENTS:
A single megasporaphyll consists of two types of scales:
(a) a large woody ovuliferous scale or seminiferous scale bearing two ovules on the adaxial surface, and
(b) a bract scale or cone scale on the abaxial surface (Fig. 1.63B, C).
Initially, the ovuliferous scale is much smaller than that of bract scale, but after pollination it becomes larger than the bract scale. The ovuliferous scale is a thick, large, woody, roughly triangular and brownish structure. Its upper thick exposed part is known as apophysis.
ADVERTISEMENTS:
In the mature cone, the tip of the apophysis becomes the ‘umbo’ (Fig. 1.63C). There are two separate vascular traces, one supplies to the ovuliferous scale and the other to the bract scale (Fig. 1.63B). There is no separate vascular trace for ovule.
The development of megasporangium (ovule) is of eusporangiate type i.e., an ovule develops from a group of superficial cells of the ovuliferous scale.
Ovule:
ADVERTISEMENTS:
The ovules of Pinus are anatropous, unitegmic and crassinucellate (Fig. 1.64). The single integument is free from the nucellus except at the chalazal end. There is a fairly broad micropylar tube which becomes inwardly curved during pre-pollination stages and becomes outwardly curved at the time of pollination.
The integument is three-layered, the outer fleshy, the middle stony and the inner fleshy.
Megasporogenesis:
A hypodermed cell in the nucellar tissue at the micropylar end is differentiated into an archesporial cell. It divides periclinally to form an upper parietal cell and a lower megaspore mother cell. The parietal cell further divides to form tapetal layer. The megaspore mother cell undergoes meiotic division to form a linear tetrad of four megaspores.
The outer three megaspores degenerate, while the lowermost megaspore becomes functional (Fig. 1.66A). The upper free opening of the integument forms the micropyle and a concavity in between the integument and nucellus in the upper part of the ovule forms the pollen chamber. After pollination the pollen grains are stored in the pollen chamber and further development of pollen grains takes place in the nucellar tissue.
Morphological Nature of Ovuliferous Scale:
There is a great controversy regarding the morphological nature of ovuliferous scale and several hypotheses have been put forward.
Some of the earlier theories which have only a historial values are briefly discussed:
1. According to Robert Brown (1827), the ovuliferous scale represents an open foliar carpel bearing naked ovules, present in the axil of bract scale.
2. According to Schleiden (1839), the ovuliferous scale represents an axillary placenta which is situated in the axil of an axillary leaf (bract scale).
ADVERTISEMENTS:
3. According to Alexander Brown (1842), the ovuliferous scale is equivalent to the first two leaves of an axillary shoot which had fused.
4. According to van Tieghem (1869), the ovuliferous scale represents a single leaf branch, present in the axil of a leaf (bract).
5. According to the foliar theory of Delpino (1889), both the ovuliferous scale and the bract scale are the parts of a tripartite bract, where the two lateral fertile lobes of the bract were fused to form ovuliferous scale and the median sterile part formed the bract scale.
6. According to ligular or excrescence theory of Sachs (1882) and Eichler (1889), the female cone represents a simple flower where the cone axis is equivalent to receptacle or thalamus and bract scale to free carpels. The ovuliferous scale represents an outgrowth of the carpel (bract scale) as in ligule of Selaginella or the placenta of angiosperms.
7. According to brachyblast theory of Braun, the female cone is equivalent to an inflorescence, where ovuliferous scale represents a determinate axillary shoot bearing two fertile leaves with a single ovule on the dorsal surface of each leaf.
8. Modern hypothesis: On the basis of the comparative studies of the fossil Cordaitales and Voltziales members, Florin (1951) introduced a terminology ‘seed-cone complex’ for ovuliferous scale and bract scale, According to Florin, the female cone of Pinus represents an inflorescence where cone axis is the peduncle, bract scale is a true bract and the ovuliferous scale is a rudimentary female flower i.e., a modified reproductive shoot.
This hypothesis has been substantiated by fossil evidences. The occurrence of several Upper Carboniferous to Triassic seed-cone complex in the members of Cordaitales and Voltziales supports the above hypothesis (Fig. 1.56A-D). In Cordaianthus, Emporia and Ernestiodendron of Upper Carboniferous, the cone axis bears several secondary female reproductive shoots in the axils of bracts.
Each secondary shoot consists of many spirally-arranged sterile and fertile (with terminal ovule) scales (showing radial symmetry).
The further evolution took place in the Triassic Voltziales like Glyptolepis, Wallchiostrobus, etc. where the scales were planated (showing bilateral symmetry) and tangentially fused to form a rudimentary ovuliferous scale following Telome hypothesis. Thus, it confirms that the ovuliferous scale is a modified reduced secondary female reproductive shoot and bract scale is actually a reduced bract.
Gametophyte of Pinus:
The spore is the first phase of gametophyte generation. The microspore or pollen grain represents the male gametophyte, while the megaspore represents the first stage of female gametophyte which develops into a female gametophyte.
i. Development of Male Gametophyte before Pollination:
The basic pattern of the development of male gametophyte of Pinus is similar to that of Ginkgo. The pollen grains undergo endosporic development. The pollen nucleus divides mitotically to produce a small lens-shaped first prothallial cell towards the proximal end and a large central cell on the distal end (Fig. 1.65A).
The central cell again cuts off a second prothallial cell and an antheridial initial (Fig. 1.65B). Both the prothallial cells are ephemeral and the second prothallial cell remains attached to the first prothallial cell.
The antheridial initial divides to form a small antheridial cell and a large tube cell (Fig. 1.65C). The pollen grains are released from the microsporangium at the 4- celled stage (2 prothallial cells, an antheridial cell and a tube cell).
ii. Development of Male Gametophyte after Pollination:
After pollination, the 4-celled pollen stores in the pollen chamber and remains ungerminated for about 11 months. The pollen develops the next spring. The tube cell of the pollen comes out through the pollen aperture in the form of a pollen tube. The pollen tube proceeds towards the archegonium, penetrating the nucellar tissue of the ovule. The antheridial cell within the pollen tube divides to form a stalk cell and a spermatogenous (body) cell (Fig. 1.65D).
The spermatogenous cell divides to form two male nuclei just prior to fertilisation (Fig. 1.65E). The male nuclei are actually the male gametes which are non-motile and ephemeral.
iii. Development of Female Gametophyte:
The female gametophyte of Pinus develops from the functional megaspore which enlarges considerably (Fig. 1.66A). The nucleus of the megaspore divides mitotically forming a large number of nuclei unaccompanied by wall formation.
The number of free nuclei is constant for a particular species, say for example, it is 2,000 for P. gerardiana and 2,500 for P. roxburghii and P. wallichiana. With the increase in size, the megaspore develops a vacuole at the centre which forces by cytoplasm along with nuclei towards the periphery.
Thus, the nuclei lie in a thin film of cytoplasm around the vacuole (Fig. 1.66B). Thereafter, the cell wall formation starts in a centripetal fashion, from periphery inwards. At this stage, numerous radially elongated multinucleate tube-like cells called alveoli are formed and the wall formation takes place through alveoli. Each alveolus containing a nucleus at its mouth directs its growth.
Then, cross-walls are laid down on each alveolus to form uninucleate cells. In this way, the entire gametophyte becomes cellular and the tissue thus formed represents endosperm or female prothallus (Fig. 1.64).
iv. Development of Archegonia:
The development of archegonia in Pinus is similar to that of Ginkgo. Two to four cells of the female gametophyte at the micropylar end enlarge in size and have dense cytoplasm and prominent nuclei. These cells function as archegonial initials. Each archegonial initial divides periclinally to form an outer small primary neck initial and a large central cell.
The primary neck initial divides by two vertical walls at right angles to each other forming a neck of four cells. Thus, the four neck cells are arranged in a single tier as in P. roxburghii and P wallichiana. However, in P. rigida, P. austriaca the four neck cells again divide transversely to form eight cells which are arranged in two tiers. The central cell enlarges very rapidly and its cytoplasm becomes vacuolated (Fig. 1.66C).
The nucleus of the central cell divides into an upper ephemeral ventral canal cell and a large egg cell. A nutritive layer called archegonial jacket is differentiated around the archegonium. The nucellar tissue above the archegonia disorganises to form an archegonial chamber.
v. Pollination:
Pinus is anemophilous i.e., wind-pollinated. The pollen grains are dispersed and remain suspended in the air for some time. At the same time, the nucellar beak in the ovule disorganises forming a viscous sugary liquid containing glucose, fructose and sucrose. This fluid comes out in a cyclic phenomenon (24 hr. cycle) through the micropyle in the form of a pollination drop either at night or in the early hours of morning. The pollen grains are caught in the pollination drop and are collected in the pollen chamber as a result of drying off the fluid. The mouth of the micropyle is then sealed from the outer environment.
vi. Fertilisation:
The fertilisation takes place after one year of pollination. The pollen tube enters the tip of the archegonium by forcing itself between the cells of the nucellus. The pollen tube wall is disintegrated by the enzymes secreted from the egg and eventually two male nuclei are released. One of the male nuclei fuses with the egg cell and thus a zygote is formed.
vii. Development of Proembryo:
The zygote nucleus divides by two mitotic divisions forming four nuclei which move to the base of zygote (Fig. 1.67A). All the four nuclei are arranged in one plane and only two nuclei are thus visible in lateral view. A synchronous division gives rise to eight nuclei arranged in two tiers of four each (Fig. 1.67B).
Thus, the upper group of four cells forms the primary upper tier (these cells have no wall towards upper side) and lower group of four cells forms the primary embryonic tier (these cells are bounded by wall all around).
The internal division in both the tiers forms four tiers of four cells each (Fig. 1.67C), and the proembryo thus consists of 16 cells. The lowest tier is known as embryonal tier, (Fig. 1.67D) which further divides to form embryo. The next tier, called suspensor tier, elongates considerably to form the embryonal suspensor.
The third tier is known as dysfunctional suspensor (earlier known as rosette tier) which shows abortive meristematic activity. The uppermost or the fourth tier is called upper tier or nutritive tier which provides nutrition.
viii. Embryogeny:
The developing embryonal cells are deeply embedded into the gametophyte by the sevenfold elongation of embryonal suspensor (Fig. 1.67E). Thus the several embryonal suspensors (designated as Es1, Es2, Es3 and so on) are formed. Proximal cells of the embryonal mass elongate unequally to form embryonal tubes.
The cells of the embryonal tier are separated from each other at the time of embryonal suspensor elongation, thus four independent embryos are formed (Fig. 1.67E). This phenomenon is known as polyembryony, because more than one embryo is formed from a zygote (Fig. 1.67F).
As the polyembryony occurs due to the splitting of a zygote, it is called cleavage polyembryony. Only a single deep-seated proembryo develops into an embryo and the growth of other embryos is arrested at different stages of development.
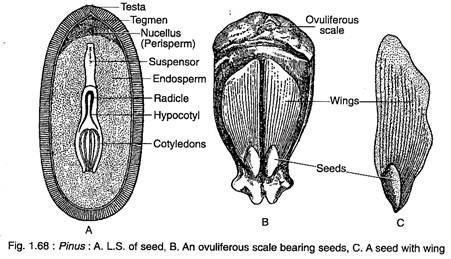
The proembryo divides transversely to form two cells which by further repeated divisions form an embryo. The embryo is comprised of 3- 18 cotyledons, a distinct epicotyl root axis and a hypocotyl shoot axis with remnants of suspensor (Fig. 1.68A). Both the two-year and three- year reproductive cycles are exemplified by Pinus species.
In two-year type, the pollination and fertilisation take place in late spring of first and second year, respectively. In three-year type the pollination takes place in spring of first year, and fertilisation in the spring of third year, a lapse of two years. The seeds shed in autumn.
Seeds of Pinus:
The seeds are endowed with a well-developed wing which is thin and papery and is easily detachable at maturity (Fig. 1.68B). The outer fleshy layer of integument and part of ovuliferous scale contribute to the wing formation (Fig. 1.68C). The seeds are usually dispersed by wind. The embryo remains embedded within the endosperm.
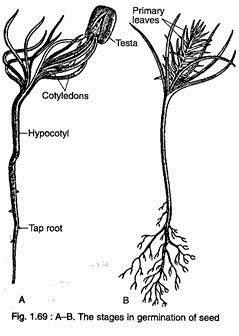
The seeds of Pinus remain viable for a long time. The germination of seed is epigeal (Fig. 1.69A, B).
Origin and Relationship of Pinus:
The conifers are large and diversified group of extant gymnosperms. The Pinaceae is the largest family of the modern conifers. The conifers had evolved from the members of Permo-Carboniferous Voltziales, commonly known as “transition conifers”. They reached their climax in mid-Mesozoic forming extensive forests in Northern Europe.
Among the living families, Pinaceae and Araucariaceae are more primitive, probably evolved during the Triassic age. On the other hand, Cupressaceae and Cephalotaxaceae are comparatively younger which have evolved probably during Upper Jurassic to Lower Cretaceous period.
Pinus is the large and well-representative genus of the family Pinaceae. It indicates relationship with Cycadales, Ginkgoales and Cordaitales.
Relation to Cycadales:
The similarities between Pinus and Cycadales are:
i. The stem anatomy shows a broad pith, large cortex and centripetal wood.
ii. The presence of haplocheilic, sunken stomata in leaves.
iii. The presence of leaf sclerenchyma.
iv. The seeds are with three-layered integument.
v. The presence of free-nuclear divisions in the development of female gametophyte.
Relation to Ginkgoales:
The resemblance between Pinus and Ginkgoales are:
i. The plants are profusely branched showing excurrent habit.
ii. The shoots are dimorphic, bearing long shoots and dwarf shoots
iii. The wood is pycnoxylic.
iv. The leaves are with haplocheilic sunken stomata.
v. The mature wood shows pitting and Bars of Sanio.
Relation to Cordaitales:
Conifers have derived from the members of Voltziales which are considered as ‘transition conifers’ between Cordaitales and Conifers. Thus, Pinus shows striking similarities with Cordaitales.
These include:
i. The plants are tall-branched trees.
ii. The leaves are sample with paralled veins.
iii. The presence of sclerenchymatous hypodermis in the leaves.
iv. The presence of pycnoxylic wood.
v. The pollen grains are winged.
vi. The ovules are bilaterally symmetrical.
Figure 1.70 shows the life cycle of Pinus: