ADVERTISEMENTS:
In this essay we will discuss about:- 1. Definition of Osmosis 2. Types of Osmosis 3. Osmotic Pressure 4. Factors 5. Importance 6. Osmotic Relations of Plant Cells.
Contents:
- Essay on the Definition of Osmosis
- Essay on the Types of Osmosis
- Essay on the Osmotic Pressure (O.P.)
- Essay on the Factors of Osmosis
- Essay on the Importance of Osmosis
- Essay on the Osmotic Relations of Plant Cells
Essay # 1. Definition of Osmosis:
ADVERTISEMENTS:
(i) Diffusion of water from its pure state or dilute solution into a solution or stronger solution when the two are separated by a semipermeable membrane is termed as osmosis.
(ii) The movement of water from its higher chemical potential (found in pure state or dilute solution) to its lower chemical potential (found in solution or stronger solution) without allowing the diffusion of solute by means of a semipermeable membrane is called osmosis. The chemical potential of water is also called water potential.
(iii) Osmosis is movement of solvent or water molecules from the region of their higher diffusion pressure or free energy to the region of their lower diffusion pressure or free energy across a semipermeable membrane.
The direction and rate of osmosis depend upon the sum of two forces, pressure gradient (gradient of Ѱp) and concentration gradient (gradient of Ѱs). The net force or gradient is determined by the difference in the water potentials of solutions separated by a semipermeable membrane.
ADVERTISEMENTS:
A solution which can cause an osmotic entry of water into it is said to be osmotically active solution. It possesses a low water potential. Diffusion of water into it will continue across the separating membrane till an equilibrium is reached. At equilibrium water potential becomes equal on both sides of the membrane.
Osmosis is a special type of diffusion of water that occurs through a semipermeable membrane.
Explanation:
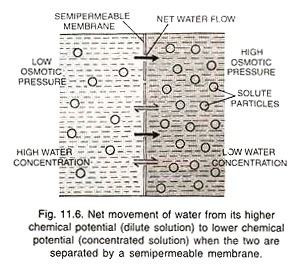
Solute particles decrease the chemical potential of water by decreasing the mole fraction of water. In osmosis, a semipermeable membrane separates (say) dilute solution A and concentrated solution B. Solute particles cannot pass through the semipermeable membrane. Water molecules are in random motion. They strike the semipermeable membrane on both the sides and pass through the same (Fig. 11.6).
Since more free water molecules are present on the side of dilute solution A, more of them pass through the membrane to enter the solution В as compared to the reverse flow. There is, therefore, a net diffusion of water from its higher chemical potential (dilute potential) to its lower chemical potential (concentrated solution).
Essay # 2. Types of Osmosis:
Osmosis is of two types:
(i) Endosmosis:
ADVERTISEMENTS:
The osmotic entry of water into a cell, organ or system
(ii) Exosmosis:
The osmotic withdrawal of water from a cell, organ or system.
Essay # 3. Osmotic Pressure (O.P.):
ADVERTISEMENTS:
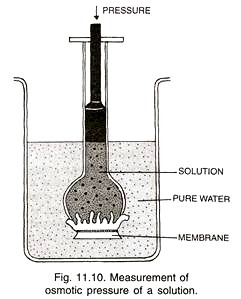
It is maximum pressure which can develop in an osmotically active solution when it is separated from its pure solvent by a semipermeable membrane under ideal conditions of osmosis that do not allow dilution of solution. Osmotic pressure is also defined as the pressure required to completely stop the entry of water into an osmotically active solution across a semipermeable membrane (Fig. 11.10).
It is measured in atmospheres, bars or Pascal’s. Osmotic pressure is numerically equal to osmotic potential (= solute, potential, Ѱs) but while osmotic potential has a negative value, osmotic pressure (n, pi) has a positive value, (Ѱs = – π). The instrument used for measuring osmotic pressure is called osmometer, e.g., Berkeley and Hartley’s osmometer, Pfeffer s osmometer.
Aquatic plants have an osmotic pressure of 1-3 atm, mesophytes 5—15 atm while in xerophytes it lies between 10-30 atm but goes up to 60 atm under drought conditions. Upper leaves have more osmotic pressure than the lower leaves. Seeds may develop an osmotic pressure of 100 atm. Halophytes have the maximum osmotic pressure with Atriplex confertifolia showing an O.P. of 202. 4 atm.
ADVERTISEMENTS:
Reverse Osmosis:
It is the expulsion of pure water from a solution through a semipermeable membrane under the influence of pressure higher than the O.R of the solution. Reverse osmosis is used in removing salts from saline water as well as extra-purification of water.
Essay # 4. Factors Controlling Osmosis:
Presence of a perfectly semipermeable membrane is a must for the operation of osmosis.
ADVERTISEMENTS:
Osmosis is driven by two other factors:
(i) Concentration of dissolved solute on the two sides of semipermeable membrane,
(ii) Difference in pressure.
In thistle funnel experiment there is no indefinite passage of water from beaker into the funnel despite the fact that osmotic potential of the solution continues to be negative as compared to the chemical potential of pure water. It is because after some distance the raised column of solution exerts sufficient pressure over the semipermeable membrane as to balance the chemical potential of pure water.
Osmotic Concentrations:
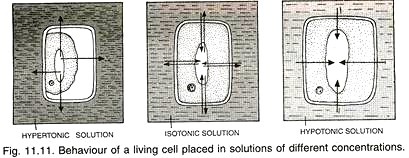
A solution having low osmotic concentration (hence low osmotic pressure but less negative solute potential) as compared to another solution is known as hypotonic solution. A solution having high osmotic concentration (hence high osmotic pressure but more negative solute potential) as compared to another solution is termed as hypertonic solution.
ADVERTISEMENTS:
The two solutions with the same concentration or pressure or potential are named as isotonic solutions. External hypotonic solution will cause endosmosis while hypertonic solution results in exosmosis. There is no change if the external solution is isotonic (Fig. 11.11). Such a flaccid cell will allow movement of water in both the directions.
Essay # 5. Importance of Osmosis:
(1) Entry of soil water into root is carried out by osmosis.
(2) Osmosis performs cell to cell movement of water.
(3) Living cells remain distended or turgid only by the osmotic entry of water into them.
ADVERTISEMENTS:
(4) Various cell organelles like mitochondria and chloroplasts will collapse if they are not able to maintain a proper osmotic concentration.
(5) 70% of cell water is held in vacuoles. It has come from outside through endosmosis and is kept in its place due to osmotic concentration of solutes dissolved in it.
(6) The soft organs like leaves, flowers, fruits and young stems are able to keep themselves stretched and swollen due to turgidity of their cells which is dependent upon osmosis.
(7) Osmosis plays a key role in the growth of radicle and plumule during the germination of seeds.
(8) Many plant movements like the folding and drooping of leaves in Mimosa are brought about by osmosis.
(9) The stomata open and close only in response to increase or decrease of the osmotic pressure of the guard cells in relation to nearby epidermal cells.
(10) A high osmotic pressure has been found to protect the plants against drought and frost injury. Seeds and spores are similarly able to pass through the unfavourable periods due to high osmotic pressure (or low solute potential).
Water Potential:
It is the difference in the free energy or chemical potential per unit molal volume of water in a system and that of pure water at the same temperature and pressure. Chemical potential of pure water at normal temperature and pressure is zero. In solutions the value of water potential is always negative or less than zero. It is represented by greek letter Ѱ (psi) or more accurately Ѱw.
The value of Ѱw is measured in bars, pascals or atmospheres. Water always moves from the area of high water potential or high energy to the area of low water potential or low energy, i.e., from less negative potential (e.g., -1000 KPa) to more negative potential (e.g., -2000 KPa).
If two systems having water are in contact, more random movement of water molecules in the system having higher water potential or more energy will result in their net movement towards the system with low energy or low water potential, e.g., soil and air, cell and solution. The movement of a substance from an area of higher free energy to area of lower free energy is called diffusion.
Water potential or Ѱw is the sum total of Ѱs and Ѱр.
Ѱw = Ѱs + Ѱp
Ѱs designates the effect of solute on the free energy of water while Ѱp indicates the effect of pressure on the same. However, another force which has a negative value and which decreases the water potential is matric potential (Ѱm).
It is reduction in free energy of water when the latter comes to form thin surface layers adsorbed over colloidal particles. It is appreciable in case of dry seeds, young cells and dry soils. However, in case of mature cells and hydrated cell walls the effect of matric potential is negligible.
Osmotic or Solute Potential (Ѱs):
It is the decrease in the chemical potential of pure water due to the presence of solute particles in it. It is a colligative property of solute and is dependent upon the number of solute particles and not upon the nature of solute.
Solute particles reduce the free energy of water by diluting it, increasing entropy, reducing vapour pressure, raising boiling point and lowering freezing point. Its value is calculated by the following formula
OP or SP = C x R x T
where С is the concentration of solute particles in moles per litre (one mole or molecular weight in grams = 6.02 x 1023 particles or molecules), R is gas constant with a value of 0.083 (0.082 if in atmospheres) and T is the temperature in absolute degrees.
Thus osmotic potential of a molar solution of a nonelectrolyte like sucrose at 0°C or 273°A and 20°C or 293°A can be calculated as below:
at 0°C =1 x 0.083 x 273 =22.7 bars or 22.4 atm (as negative)
at 20°C =1 x 0.083 x 293 =24.3 bars or 24 atm. (as negative)
In case of electrolytes, the degree of ionisation is taken into consideration because osmotic or solute potential depends upon the number of particles (here as ions and molecules) and not upon the number of molecules alone.
In case of strong electrolytes the osmotic potential may be almost double or triple as compared to nonelectrolytes. For example, 0.1N sucrose solution has an osmotic potential of—2.3 bars while 0.1N sodium chloride solution has Ѱs value of -4.5 bars.
Pressure Potential (Ѱp) or Hydrostatic Pressure:
It is the pressure which develops in an osmotic system due to osmotic entry or exit of water from it. A positive pressure develops in a plant cell or system due to entry of water into it.
Positive hydrostatic pressure is also called turgor pressure. Loss of water produces a negative hydrostatic pressure or tension. It develops in xylem due to loss of water in transpiration. This is very important in transport of sap over long distances in plants.
Positive hydrostatic pressure or turgor pressure is the pressure which develops in the confined part of an osmotic system due to osmotic entry of water into it. Due to turgor pressure the protoplast of a plant cell will press the cell wall to the outside. The cell wall, being elastic, presses the protoplast with an equal and opposite force.
The force exerted by the cell wall over the protoplast is called wall pressure (WP). Normally wall pressure is equal and opposite to turgor pressure except when the cell becomes flaccid. The values of these two opposing forces continue to rise till the cell becomes fully swollen or turgid. At this time the value of wall or turgor pressure becomes equal to osmotic potential, Ѱp = Ѱs.
Turgor pressure has number of important functions:
(i) It keeps the cells and their organelles stretched. This is essential for proper functioning of a cell.
(ii) It provides support to non-woody tissues like parenchyma.
(iii) Turgor pressure is essential for cell enlargement during growth.
(iv) It keeps the leaves fully expanded and properly oriented to light.
Flowers, young stems and other softer organs are able to maintain their form due to turgidity or TP. In case of loss of turgidity, the shoots droop down and the leaves show wilting.
Wilting is observed in many plants during summer noon. In wilting the individual cells of leaves and other softer parts become flaccid due to loss of water from their interior. Plants gain their turgidity during night because of continued absorption of water from the soil.
If the soil does not obtain water periodically (i.e., the soil is deficient in water), the recovery may be only partial or it may not occur at all. The latter condition is known as permanent wilting. The plants are soon killed. Temporary wilting reduces growth and decreases the yield of plant.
(v) Many plant movements are produced due to reversible turgor changes in the cells. Sleep movements (nyctinasty) of bean leaves and shock movements (seismonasty) of Sensitive Plant (Mimosa pudica) are produced due to turgor changes in the cells of their pulvini.
Regular turgor changes in the leaflet bases cause rhythmic autonomic jerky movements in the lateral leaflets of Indian Telegraph Plant, Desmodium gyrans (Fig. 11.12).
(vi) The opening and closing of stomata are caused by gain and loss of turgidity by their guard cells. They are hence often called “turgor operated valves.”
(vii) Turgor pressure (pressure potential or hydrostatic pressure) keeps a check on the excessive entry of water into cells.
(viii) Autochory of some fruits is dependent upon release of turgor pressure.
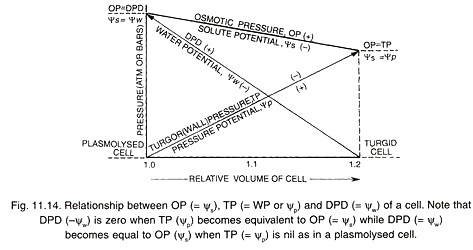
Essay # 6. Osmotic Relations of Plant Cells (Fig. 11.13):
A plant cell functions as an osmotic apparatus or osmotic system. It is bathed in a water medium or lies in contact with other cells having water. A typical plant cell has a permeable elastic wall, a semipermeable membrane (plasma lemma or plasma membrane alone or along with cytoplasm and tonoplast) and an osmotically active solution called cell sap (contained in the central vacuole).
The osmotically active cell sap has an osmotic potential (Ѱs or OP). It causes the osmotic entry of water which develops a turgor pressure (TP) or pressure potential (Ѱp). The osmotic status of the cell is written as, Ѱw = Ѱs + Ѱp or DPD = OP – TP (= WP). Ѱs or solute potential is a negative force. It causes entry of water into the cell.
As a result a cell protoplast swells and develops the positive force of Ѱp. Ѱp is kept under check by wall pressure. The cell where wall is absent cannot counteract pressure potential. It will continue to absorb water due to Ѱs till it bursts. It is because of this fact that the animal cells are not bathed in water but instead are surrounded by nearly isotonic tissue fluid.
Being a positive force, Ѱp opposes the entry of water into the cell.
(a) Rise in Turgidity:
A flaccid cell placed in hypotonic solution will absorb water. The cell swells up. The value of turgor pressure rises while that of solute potential becomes slightly less negative. As the cell becomes fully turgid, the value of turgor pressure becomes equal to that of solute potential (Ѱs) so that water potential (Ѱw) or DPD becomes either zero or equal to that of external hypotonic solution.
Ѱw = Ѱs + ѰP = 0
Though there is no net movement of water between the cell and its environment, equilibrium is dynamic and not static. Equal exchange of water molecules continues between the cell and its environment.
(b) Loss of Turgidity:
If plant cell happens to be bathed in hypertonic solution, it loses water through the process of exosmosis. The loss of water is first from cytoplasm and then central vacuole. As a result, the protoplast is reduced in size. This decreases turgor pressure or pressure potential and corresponding wall pressure.
Solute potential becomes slightly more negative due to loss of water. The cell attains a minimum size when turgor pressure is zero. If the external solution does not cause any further exosmosis, the value of its solute potential will be equal or isotonic to solute potential of the cell.
Ѱw = Ѱs + ѰP = or Ѱw = Ѱs
A cell which is deficient in turgor is called flaccid. A flaccid cell kept in isotonic solution will show equal flow of water into and out of the cell. In case exosmosis continues, the protoplast shrinks from the cell wall. The phenomenon is called plasmolysis.
(c) Water Relations between Adjacent Cells (Fig. 11.15):
Cells gain or lose water among themselves on the basis of their water potential (or DPD) and not their solute or osmotic potentials. A cell having more negative osmotic potential and high turgor pressure can lose water to a cell having less negative osmotic potential provided it has still lower turgor pressure.