ADVERTISEMENTS:
Here is an essay on ‘Cancer Cell Proliferation’ for class 11 and 12. Find paragraphs, long and short essays on ‘Cancer Cell Proliferation’ especially written for college and medical students.
Essay on Cancer Cell Proliferation
Essay Contents:
ADVERTISEMENTS:
- Essay on the Introduction to Cancer Cell Proliferation
- Essay on the Features of Cancer Cell Proliferation
- Essay on Growth Factors and the Cancer Cell Cycle
- Essay on Traits Affecting Cancer Cell Proliferation
- Essay on the Role of DNA in Cancer Cell Proliferation
- Essay on Immune System and Cancer Cell Proliferation
- Essay on Molecular Changes in Cancer Cell Proliferation
1. Essay on the Introduction to Cancer Cell Proliferation:
Cancer cells exhibit a number of unusual properties that distinguish them from normal cells. One key property is the ability of cancer cells to proliferate in an uncontrolled fashion, thereby leading to a progressive accumulation of dividing cells without regard to the needs of the body as a whole.
In reality, uncontrolled proliferation is not a single discrete property but rather a collection of traits that together allow cancer cells to escape from the usual restraints on cell proliferation. The net result is that cancer cells circumvent the mechanisms designed to ensure that cells divide only when (and where) new cells are needed.
ADVERTISEMENTS:
The normal mechanisms for controlling cell proliferation and see how they behave in cancer cells. Through such a discussion it will become apparent that cancer cells exhibit a distinctive collection of abnormal properties, and while no single property is necessarily seen in every cancer cell, as a group these traits contribute to the “profile” of a typical cancer cell.
Although cancer can result from abnormal proliferation and development of tumours, the key issue is, whether the tumour is benign or malignant. The malignant tumour is capable of invading normal tissues, spreading in the body via circulatory or lymphatic systems and establishing secondary tumours (metastasis).
Most cancers fall into one of three main groups: carcinomas which arise from epithelial cells; sarcomas are solid tumours of connective tissues such as muscle, bone, cartilage and fibrous tissue; leukemia’s and lymphomas arise from blood forming cells and from cells of the immune systems, respectively. Further classification of tumours is based on tissue of origin and type of cell involved in malignancy.
A key feature of tumours is their development from single cells that begin to proliferate abnormally, hence clones of cells are present in tumours. The development of malignancy in cell clones is a multistep process accompanied by a series of changes in the cells. In general, cancer is considered as a multistep process involving mutation and selection of cells with progressively increasing capacity for proliferation, invasion and metastasis.
The first step, tumour initiation involves genetic alteration leading to abnormal proliferation of a single cell. Tumour progression continues as additional changes take place in tumour cell population. Metastasis occurs when tumour cells invade other organs and establish secondary sites of malignancy.
Substances that can cause cancer are called carcinogens, and include many agents such as radiation, chemicals, viruses and many more. Radiation and chemicals can initiate cancer by damaging DNA and inducing mutations in key target genes.
Some carcinogens contribute to cancer development by stimulating cell proliferation, rather than by inducing mutations. Such compounds are designated tumour promoters because by inducing increased cell division they produce a proliferative cell population during early stages of tumour development.
Classic examples are the phorbol esters that stimulate cell proliferation by activating protein kinase C. Hormones, particularly estrogens, are important as tumour promoters in the development of some human cancers. For example, the uterine epithelium responds to excess estrogen and increases the likelihood of development of endometrial cancer. Some viruses also induce cancer in experimental animals and humans, such as liver cancer and cervical carcinoma in humans.
Cancer cells display features that distinguish them from their normal counterparts. Cancer cells have abnormalities in the mechanisms that regulate normal cell proliferation, differentiation and survival. In culture, cancer cells can be distinguished from normal cells in displaying density- dependent inhibition of cell proliferation. Normal cells continue to divide until they reach a finite cell density.
ADVERTISEMENTS:
They then stop dividing and become quiescent, arrested in the G0 stage of the cell cycle. The proliferation of cancer cells is independent of density-dependent inhibition. Such cells do not respond to signals that cause normal cells to cease proliferation.
Instead, tumour cells continue to grow to high cell densities in culture, this behaviour corresponds with their uncontrolled proliferation in vivo. The proliferation of many normal cells is controlled in part, by polypeptide growth factors. Cancer cells have reduced requirement for extracellular growth factors.
Both in vitro and in vivo, growth factor requirements of cancer cells are reduced contributing to unregulated proliferation of tumour cells. In some cases cancer cells produce growth factors that stimulate their own proliferation. Such an abnormal production of a growth factor leads to continuous stimulation of cell division, called autocrine growth stimulation, and makes cancer cells less dependent on growth factors from normal sources.
2. Essay on the Features of Cancer Cell Proliferation:
ADVERTISEMENTS:
The concept of uncontrolled proliferation is unique to multicellular organisms. In most single-celled organisms, such as bacteria or yeast, the presence of sufficient nutrients in the surrounding environment is the main factor that determines whether cells will grow and divide.
The situation is reversed in multicellular organisms; cells are usually surrounded by nutrient-rich extracellular fluids, but the organism as a whole would be quickly destroyed if each cell were to continually grow and divide just because it had access to adequate nutrients. Cancer is a potentially lethal reminder of what happens when cell proliferation continues unabated without being coordinated with the needs of the organism as a whole.
i. Cancer Cells Produce Tumors when Injected into Laboratory Animals:
It is the loss of normal growth control that causes cancer cells to produce a continually growing mass of tissue—in other words, a tumor—but uncontrolled growth does not mean that tumor cells always divide more rapidly than normal cells. Tumors can grow slowly, quickly, or somewhere in between. The distinctive feature of tumor growth is not the speed of cell division but its uncontrolled nature.
ADVERTISEMENTS:
In contrast to the proliferation of normal cells, where cell division and cell differentiation are kept in proper balance, this finely tuned arrangement is disrupted in tumors and cell division is uncoupled from cell differentiation, thereby leading to a progressive increase in the number of dividing cells.
To determine experimentally whether a particular cell behaves in this way, the cell must be injected into an appropriate host organism to see if a tumor will develop. Experiments involving animal cells are fairly straightforward because the cells can simply be injected into animals of the same genetic type.
The situation with human cells is more complicated. Injecting human cancer cells into other humans for testing purposes would be unethical, and using standard laboratory animals is not reliable: An animal’s immune system is likely to reject human cells because they are of foreign origin.
One way around this obstacle is to inject human cells into mutant strains of mice whose immune systems are unable to attack and destroy foreign cells. When human cancer cells are injected into such immunologically deficient animals, the cells will usually grow into tumors without being rejected.
ADVERTISEMENTS:
ii. Cancer Cells Exhibit Decreased Density-Dependent Inhibition of Growth:
Although studying cancer cells in intact organisms is useful for investigating some of the properties of malignant tumors, issues related to the control of cell proliferation are often easier to investigate in cells grown under artificial laboratory conditions. In such cell culture studies, cancer cells are isolated from a tumor and placed in a defined growth medium containing nutrients, salts, and other molecules required for cell growth.
The main reason for studying cells in culture is that this method allows the cells to be observed under carefully controlled conditions where their behavior can be assessed without the complicating effects of secondary factors present in an intact organism.
When most types of normal cells are placed in a culture vessel (test tube, bottle, flask, or dish) and then covered with an appropriate growth medium, they divide until the surface of the container is covered by a single layer of cells. When this monolayer stage is reached, cell movements and cell division both tend to stop.
In the early 1950s, Michael Abercrombie and Joan Heaysman introduced the term “contact inhibition” to refer to the decrease in cell motility that occurs when cells make contact with one another in culture. The same term has also been used to refer to the inhibition of cell division that takes place when culture conditions become crowded.
Because of the confusion that can result from the double meaning of this term, the phrase density-dependent inhibition of growth is now routinely used when referring to the inhibition of cell division that occurs in crowded cultures.
ADVERTISEMENTS:
In contrast to normal cells, cancer cells do not stop dividing when they reach the monolayer stage. Instead, they continue to divide and gradually pile up on top of one another, forming multilayered aggregates (Figure 1). In other words, cancer cells are less susceptible to density- dependent inhibition of growth than are their normal counterparts.
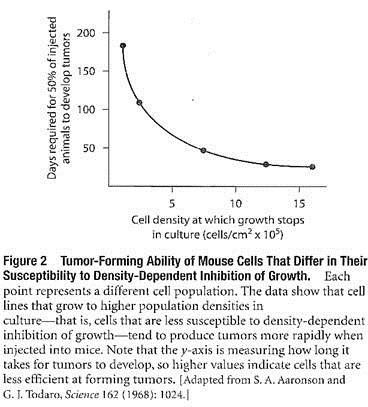
The relationship between the tendency of cancer cells to grow to high population densities in culture and their ability to form tumors has been investigated using cancer cells that differ in their susceptibility to density- dependent inhibition of growth.
Cells that are very sensitive to density-dependent inhibition can be produced by growing cells under un-crowded conditions; every time the population density increases and crowding is imminent, the cells are simply diluted and transferred to a new culture flask.
Cells obtained in this way will not grow to high population densities in culture. Alternatively, cell populations that are insensitive to density-dependent inhibition of growth can be produced by consistently growing cells in overcrowded conditions. Such cell populations become less susceptible to density-dependent inhibition of growth, reaching much higher population densities before cell division stops.
When these different cell populations are tested for their ability to produce tumors in mice, tumor-forming ability is found to be directly related to the loss of density- dependent growth control; in other words, cells capable of growing to the highest population densities in culture are most effective at forming tumors in animals (Figure 2).
iii. Cancer Cell Proliferation is Anchorage-Independent:
Another way in which the proliferation of cancer cells differs from that of normal cells involves the requirement for anchorage. Most normal cells will not proliferate if they are put in a liquid growth medium and shaken or stirred to keep them in suspension, nor will they proliferate if they are placed in a semisolid medium such as soft agar. When they are provided with an appropriate solid surface to which they can adhere, however, the cells will attach to the surface, spread out, and begin to proliferate (Figure 3).
The growth of normal cells is therefore said to be anchorage-dependent. In contrast, most cancer cells grow well not just when they are anchored to a solid surface, but also when they are suspended in a liquid or semisolid medium. The growth of cancer cells is therefore said to be anchorage-independent.
In intact organisms, the requirement that cells be anchored before they can reproduce is met by binding cells to the extracellular matrix, an insoluble meshwork of protein and polysaccharide fibers that fills the spaces between neighboring cells. Cells attach themselves to the extracellular matrix through cell surface proteins called integrins, which bind to molecules present in the matrix.
Apoptotic cell death triggered by lack of contact with the extracellular matrix is called anoikis (from the Greek word for “homelessness”). Anoikis is an important safeguard for maintaining tissue integrity because it prevents normal cells from floating away and setting up housekeeping in another tissue.
The lack of anchorage simply causes cells to commit suicide along the way. Cancer cells are not subject to this normal safeguard because they are anchorage-independent and so can spread to distant sites without self-destructing.
Considerable evidence suggests that anchorage- independent growth exhibited by cells grown in culture is related to their ability to form tumors. One set of studies involved cells with many of the traits of cancer cells, including decreased density-dependent inhibition of growth, low requirements for external growth factors, and anchorage-independent growth.
Single cells were isolated from the original population and allowed to proliferate separately, thereby creating a series of clones, which are individual cell populations each derived from the proliferation of a single cell.
Careful analysis of the clones revealed that some of them had lost one or more of the initial properties. When the ability of these clones to produce tumors in animals was compared, anchorage- independent growth was the only property consistently retained by all the clones that could produce tumors. In other words, the ability to form tumors appeared to require cells whose growth in culture is anchorage-independent.
This connection between anchorage-independent growth and tumor formation is not without its exceptions, however. Some cells exposed to cancer-causing chemicals have been found to exhibit anchorage-independent growth in culture but do not form tumors when injected into animals.
In addition, studies involving a long-term culture of mouse cells, which were anchorage-dependent and unable to form tumors in animals, showed that the cells could acquire the capacity to form tumors if they were attached to glass beads prior to being implanted in mice. Such observations indicate that despite its general association with the ability to form tumors, anchorage- independent growth in culture is not an absolute prerequisite for tumor formation.
iv. Mechanisms for Replenishing Telomeres make Cancer Cells Immortal:
One of the most striking differences between normal cells and cancer cells involves their reproductive lifespans. When normal cells are grown in culture, they usually divide for only a limited number of times. For example, human fibroblasts—a cell type whose behavior has been extensively studied—divide about 50 to 60 times when placed in culture and then stop dividing, undergo a variety of degenerative changes, and may even die (Figure 4).
Cancer cells exhibit no such limit and continue dividing indefinitely, behaving as if they were immortal. A striking example is provided by HeLa cells, which were obtained from a malignant tumor of the uterus arising in a woman named Henrietta Lacks (hence the name “HeLa” cells).
After removing the tumor in a cancer operation performed in 1951, doctors placed some of its cells in culture. The cultured cells began to grow and divide and have continued to do so for more than 50 years, dividing more than 18,000 times with no signs of stopping.
Why are cancer cells capable of reproducing indefinitely in culture, whereas most normal human cells divide no more than 50 or 60 times? The answer is related to the mechanism by which cells replicate their DNA. Each time a cell divides, its chromosomal DNA molecules must be duplicated so that a complete set of genetic instructions can be distributed to each of the two cells produced by cell division.
However, the biochemical mechanism responsible for DNA replication has an inherent limitation- The enzymes that replicate DNA are unable to copy the very end of a linear DNA molecule, perhaps the final 50 to 100 nucleotides or so.
As a result, each time a DNA molecule is replicated; it is in danger of losing a small amount of DNA at each of its two ends. If this trend were to continue indefinitely, DNA molecules would become shorter and shorter until there was nothing left, and we would not be here today!
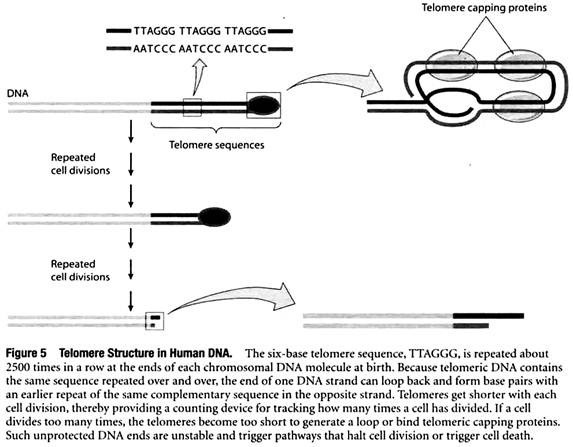
To solve this so-called end-replication problem, cells place a special type of DNA sequence at the two ends, or telomeres, of each chromosomal DNA molecule. The special DNA consists of multiple copies of a short base sequence repeated over and over again. For example, in humans the six-base sequence TTAGGG is repeated about 2500 times in a row at the ends of each chromosomal DNA molecule at birth.
These telomere sequences are protected by telomere capping proteins, and the DNA also loops back upon itself to protect the end of the chromosome even further (Figure 5). Unlike genes, whose DNA base sequences code for useful products, telomeric DNA does not code for anything but simply consists of the same six-base sequence repeated again and again.
Placing such noncoding telomere DNA at the ends of each chromosome ensures that a cell will not lose any important genetic information when DNA molecules are shortened slightly during replication.
Since telomeres get shorter with each cell division, they provide a counting device for tracking how many times a cell has divided. If a cell divides too many times, the telomeres become extremely short and are in danger of disappearing entirely. When this happens, the telomeric DNA becomes too short to bind telomeric capping proteins or generate a loop, exposing a bare end of double-stranded DNA.
Such unprotected DNA ends are very unstable and often fuse with each other, creating joined chromosomes that tend to become fragmented and separate improperly at the time of cell division.
In normal cells, such a hazardous outcome is prevented by a mechanism in which the unprotected DNA at the end of a chromosome triggers a pathway that halts cell division or triggers cell death. This pathway helps protect organisms from any inappropriate, excessive proliferation of adult cells.
But what happens with cells that must divide for prolonged periods of time, such as the germ cells that give rise to sperm and eggs or the bone marrow cells that continually produce new blood cells? Such cells prevent excessive telomere shortening by producing an enzyme called telomerase, which adds new copies of the telomeric repeat sequence to the ends of existing DNA molecules.
The telomerase-catalyzed addition of new telomere repeat sequences prevents the gradual decline in telomere length that would otherwise occur at both ends of a chromosome during DNA replication. The presence of telomerase therefore allows cells to divide indefinitely without telomere shortening.
How do the preceding considerations apply to cancer cells? If cancer cells behaved like most normal cells, which do not produce telomerase, repeated cell divisions would cause the telomeres to become unusually short and the cells would eventually be destroyed. Most cancer cells circumvent this problem by activating the gene that produces telomerase, thereby causing new copies of the telomeric repeat sequence to be continually added to the ends of their DNA molecules.
A few cancer cells activate an alternative mechanism for maintaining telomere sequences that involves the exchange of sequence information between chromosomes. By one mechanism or the other, cancer cells maintain telomere length above a critical threshold and can therefore divide indefinitely.
3. Essay on Growth Factors and the Cancer Cell Cycle:
We have now seen that cancer cells differ from most normal cells in that they grow to high population densities in culture, exhibit anchorage-independent proliferation, and divide indefinitely because they possess mechanisms for maintaining telomere length.
These traits play an important permissive role in allowing cancer cells to continue dividing, but they do not actually cause cells to divide. The driving force for ongoing proliferation can be traced to abnormalities in the signalling systems that control cell division, the topic to which we now turn.
i. Cancer Cells Exhibit a Decreased Dependence on External Growth Factors:
The cells of multicellular animals do not normally divide unless they are stimulated to do so by an appropriate signalling protein known as a growth factor. For example, if cells are isolated from an organism and placed in a culture medium containing nutrients and vitamins, they will not proliferate unless an appropriate growth factor is also provided.
Growth media are therefore commonly supplemented with blood serum, which contains several growth factors that stimulate cell proliferation. One is platelet-derived growth factor (PDGF), a protein produced by blood platelets that stimulates the proliferation of connective tissue cells and smooth muscle cells. Another growth factor in blood serum, called epidermal growth factor (EGF), is also widely distributed in tissues.
Some growth factors, such as EGF, stimulate the growth of a wide variety of cell types, whereas others act more selectively on particular target cells. Growth factors play important roles in stimulating tissue growth during embryonic and early childhood development, and during wound repair and cell replacement in adults.
For example, release of the growth factor PDGF from blood platelets at wound sites is instrumental in stimulating the growth of tissue required for wound healing.
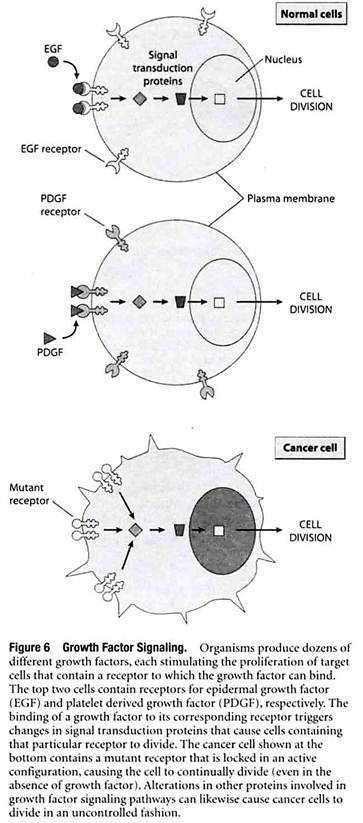
Growth factors exert their effects by binding to receptor proteins located in the plasma membrane that forms the outer boundary of all cells. Different cell types have different plasma membrane receptors and hence differ in the growth factors to which they respond (Figure 6).
The binding of a growth factor to its corresponding receptor triggers a multistep cascade in which a series of signal transduction proteins relay the signal throughout the cell, triggering molecular changes that stimulate (or occasionally inhibit) cell growth and division.
Cells will not normally divide unless they are stimulated by an appropriate growth factor, but this restraint is circumvented in cancer cells by various mechanisms that create a constant signal to divide, even in the absence of growth factors.
Some cancer cells achieve this autonomy by producing their own growth factors, thereby causing cell proliferation to be stimulated without the need for growth factors produced by other cells. Similarly, other cancer cells possess abnormal receptors that are permanently activated, causing cell division to occur whether growth factors are present or not.
Cancer cells may also produce excessive quantities or hyperactive versions of other proteins involved in relaying signals from cell surface receptors to the cell division machinery in the cell’s interior. The net effect of the preceding types of alterations is to cause the pathways that signal cell proliferation to become hyperactive or even autonomous, functioning in the absence of growth factors.
ii. The Cell Cycle is Composed of G1, S, G2, and M Phases:
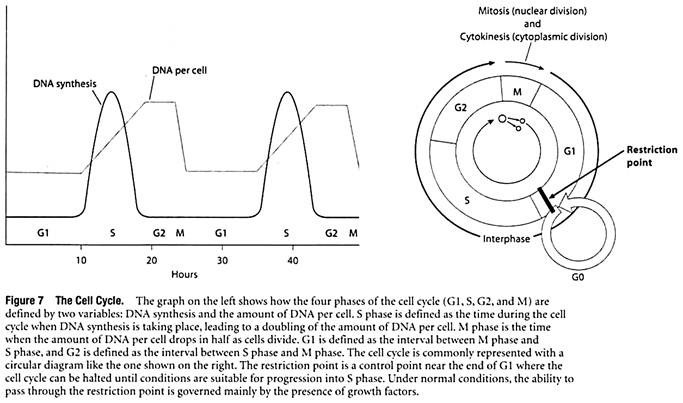
To understand how pathways activated by growth factors ultimately cause a cell to divide, it is first necessary to review the events associated with cell division. In cells that are dividing, the nuclear DNA molecules must be duplicated and then distributed in a way that ensures that the two new cells each receive a complete set of genetic instructions. In preparing for and accomplishing these tasks, cells pass through a series of discrete stages called G1 phase, S phase, G2 phase, and M phase.
The four phases are collectively referred to as the cell cycle (Figure 7).
G1—the first phase to occur after a cell has just divided—vanes the most in length. A typical G1 phase lasts about 8 to 10 hours in human cells, but rapidly dividing cells may spend only a few minutes or hours in Gl. Conversely, cells that divide very slowly may become arrested in G1 and spend weeks, months, or even years in the offshoot of G1 called the G0 phase (G zero). After completing G1, the cell enters S phase, a period of roughly 6 to 8 hours when the chromosomal DNA molecules are replicated.
Next comes G2 phase, where 3 to 4 hours are spent making final preparations for cell division. The cell then enters M phase, which takes about an hour to physically divide the original cell into two new cells. The main events of M phase include division of the nucleus, or mitosis, followed by division of the cytoplasm, or cytokinesis. The two newly formed cells then enter again into G1 phase and begin preparations for another round of cell division.
Taken together, the G1, S, and G2 phases are collectively referred to as interphase. Besides providing the time needed for a cell to make copies of its DNA molecules, interphase is also a period of cell growth. Interphase occupies about 95% of a typical cell cycle; whereas the actual process of cell division (M phase) only takes about 5%.
Overall, the time occupied by the various stages of the cycle allows a typical human cell to divide as often as once every 18 to 24 hours. However, the various cell types that make up the body differ greatly in cycle time, ranging from cells that divide very rapidly and continuously to differentiated cells that do not divide at all.
The variability observed in rates of cell division means that mechanisms must exist for regulating progression through the cell cycle. A key control point has been identified during late G1, where the cell cycle is usually halted in cells that stop dividing. For example, the division of cultured cells can be slowed down or stopped by allowing the cells to run out of either nutrients or growth factors, or by adding inhibitors of vital processes such as protein synthesis.
In such cases, the cell cycle is halted in late GI at a point referred to as the restriction point. Under normal conditions, the ability to pass through the restriction point is governed mainly by the presence of growth factors.
Cells that successfully move through the restriction point are committed to S phase and the remainder of the cell cycle, whereas those that do not pass the restriction point enter into GO and reside there for variable periods of time, awaiting a signal that will allow them to re-enter G1 and pass through the restriction point.
iii. Progression through the Cell Cycle is Driven by Cyclin-Dependent Kinases:
At the molecular level, passage through the restriction point and other key points in the cell cycle is controlled by proteins known as cyclin-dependent kinases (Cdks). Cdks are protein kinases, a term referring to a class of enzymes that regulate the activity of targeted protein molecules by catalyzing their phosphorylation (attachment of phosphate groups to the targeted proteins).
During protein phosphorylation reactions, the phosphate group is donated to the targeted protein by the high-energy compound ATP (adenosine triphosphate), which is converted to ADP (adenosine diphosphate) during the reaction. Cells contain dozens of different protein kinases, each designed to regulate the activity of a specific group of proteins by catalyzing their phosphorylation.
As the name implies, a cyclin-dependent kinase (Cdk) only exhibits protein kinase activity when it is bound to another type of protein called a cyclin. Progression through the cell cycle is controlled by several Cdks that bind to different cyclins, thereby creating a variety of Cdk- cyclin complexes.
Cyclins involved in regulating the progression from G1 to S phase are called G1 cyclins, and the Cdk molecules to which they bind are known as G1 Cdks. Likewise, cyclins involved in regulating passage from G2 into M phase are called mitotic cyclins, and the Cdk molecules to which they bind are known as mitotic Cdks. Cdk-cyclin complexes act by phosphorylating specific target proteins whose actions are required for various stages of the cell cycle.
How do Cdk-cyclin complexes ensure that passage through key points in the cell cycle only occurs at the appropriate time? In addressing this question, we will briefly consider the behavior of the mitotic Cdk-cyclin complex (mitotic Cdk bound to mitotic cyclin), which regulates passage from G2 to M phase.
Mitotic cyclin is continuously synthesized throughout interphase and gradually increases in concentration during G1, S, and G2, eventually reaching a concentration that is high enough to bind to mitotic Cdk (Figure 8).
The resulting mitotic Cdk- cyclin triggers passage from G2 into M phase by phosphorylating key proteins involved in the early stages of mitosis. For example, proteins phosphorylated by mitotic Cdk-cyclin trigger nuclear envelope breakdown, chromosome condensation, and mitotic spindle formation.
Shortly thereafter, mitotic cyclin is targeted for degradation by an enzyme called the anaphase-promoting complex and mitotic Cdk becomes inactive, triggering the exit from mitosis. During the next cell cycle, mitosis cannot be triggered until the concentration of mitotic cyclin builds up again.
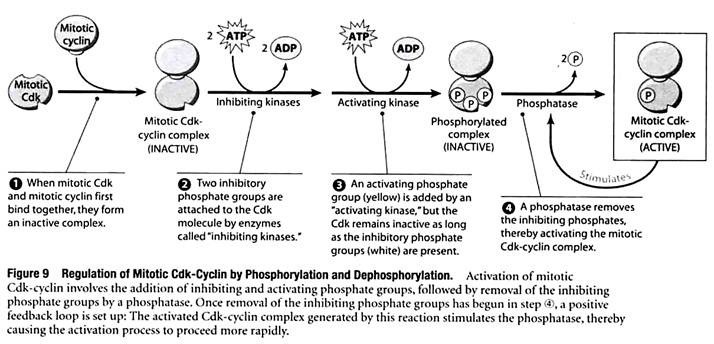
Besides being regulated by the availability of cyclins, the activity of the various Cdk-cyclin complexes is controlled by reactions in which Cdk molecules are altered by phosphorylation (addition of phosphate groups) and dephosphorylation (removal of phosphate groups).
Figure 9 illustrates how the mitotic Cdk-cyclin complex is regulated in this way:
In step ①, the binding of mitotic cyclin to mitotic Cdk creates a complex that is initially inactive. Before it can trigger passage from G2 into M phase, the complex requires the addition of an activating phosphate group to a particular amino acid of the Cdk molecule. Prior to adding this phosphate, however, inhibitory phosphate groups are first attached to the Cdk molecule at two other locations, preventing the Cdk from functioning (step ②). The activating phosphate group, highlighted with yellow in step ③, is then added. The last step in the activation sequence is the removal of the inhibiting phosphates by a specific enzyme called a protein phosphatase (step ④).
Once the phosphatase begins removing the inhibiting phosphates, a positive feedback loop is set up: The activated Cdk-cyclin complex generated by this reaction stimulates the phosphatase, thereby causing the activation process to proceed more rapidly. After being activated, the mitotic Cdk-cyclin complex triggers passage from G2 into M phase by catalyzing the phosphorylation of proteins required for the onset of mitosis.
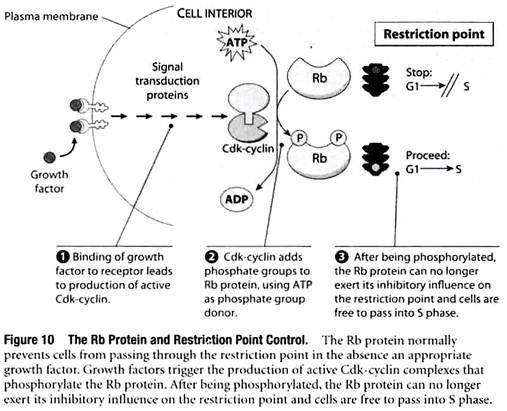
Growth Factor Signaling Pathways Act on the Restriction Point by Stimulating Phosphorylation of the Rb Protein:
Now that Cdk-cyclins have been introduced, we can explain how growth factors exert their control over cell proliferation. If normal cells are placed in a culture medium containing nutrients and vitamins but no growth factors, the cells become arrested at the restriction point. Subsequent addition of growth factors is sufficient to cause the cells to start dividing again.
How do growth factors cause G1-arrested cells to resume progression through the cell cycle? The binding of a growth factor to its corresponding cell surface receptor causes the receptor to become activated, and the activated receptor then triggers a complex pathway of reactions involving dozens of different cytoplasmic and nuclear molecules that relay the signal throughout the cell. Here we are concerned only with the question- How do these pathways impinge on the cell cycle and cause cells to pass through the restriction point and into S phase?
The answer to this question is that growth factor signaling pathways trigger the production of Cdk-cyclins that in turn catalyze the phosphorylation of target proteins required for the transition into S phase.
A key target is the Rb protein, a molecule that normally restrains cell proliferation by preventing passage through the restriction point (Figure 10). After Cdk-cyclin phosphorylates Rb, it can no longer exert this inhibitory influence and cells are free to pass through the restriction point and into S phase.
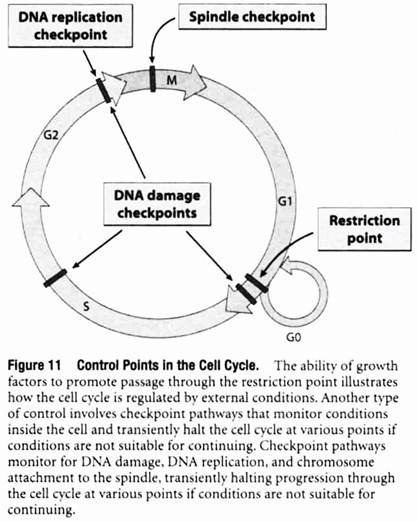
Checkpoint Pathways Monitor for DNA Replication, Chromosome-to-Spindle Attachments, and DNA Damage:
The ability of growth factors to promote passage through the restriction point by stimulating the production of Cdk-cyclins that phosphorylate Rb is just one example of how the cell cycle is controlled by external and internal factors that determine whether or not a cell should divide.
Another type of cell cycle control involves a series of checkpoint pathways that prevent cells from proceeding from one phase to the next before the preceding phase has been properly completed. These checkpoint pathways monitor conditions within the cell and transiently halt the cell cycle at various points if conditions are not suitable for continuing (Figure 11).
One such mechanism, called the DNA replication checkpoint, monitors the state of DNA replication to ensure that DNA synthesis has been completed prior to proceeding with cell division. If DNA replication is not complete, the cell cycle is halted to allow DNA replication to be finished prior to entering M phase. The existence of the DNA replication checkpoint has been demonstrated by treating cells with inhibitors of DNA synthesis.
Under such conditions the final dephosphorylation step involved in activating mitotic Cdk-cyclin (step 4 in Figure 9) is blocked through a series of events triggered by proteins associated with replicating DNA. The resulting lack of active mitotic Cdk-cyclin halts the cell cycle at the end of G2 until DNA replication is completed.
A second checkpoint mechanism, called the spindle checkpoint, acts between the metaphase and anaphase stages of mitosis, the point where the two duplicate sets of chromosomes are about to be parceled out to the two new cells being formed by the process of cell division.
At the end of metaphase, the two sets of chromosomes are normally lined up at the center of the mitotic spindle, a structure composed of microtubules that attach to the chromosomes and eventually pull them into the two newly forming cells. Before chromosome movement begins (the event that marks the beginning of anaphase), the spindle checkpoint mechanism is invoked to make certain that the chromosomes are all properly attached to the spindle.
If the chromosomes are not completely attached, the cell cycle is temporarily halted at this point to allow the process to be completed. In the absence of such a control mechanism for monitoring chromosome- to-spindle attachments, there would be no guarantee that each of the newly forming cells would receive a complete set of chromosomes.
A third type of checkpoint is used to prevent cells with damaged DNA from proceeding through the cell cycle. In this case, a series of DNA damage checkpoints monitor for DNA damage and halt the cell cycle at various points—including late G1, S, and late G2—by inhibiting different Cdk-cyclin complexes. A molecule called the p53 protein plays a central role in these checkpoint pathways.
In the presence of damaged DNA, the p53 protein accumulates and triggers cell cycle arrest to provide time for the DNA damage to be repaired. If the damage cannot be repaired, p53 may also trigger cell death by apoptosis. The ability of p53 to trigger cell cycle arrest or cell death prevents cells with damaged DNA from proliferating and passing the damage on to succeeding generations of cells.
iv. Cell Cycle Control Mechanisms are Defective in Cancer Cells:
The preceding discussion of cell cycle control mechanisms has focused largely on the behavior of normal cells. How do these principles apply to the behavior of cancer cells, which grow and divide in an uncontrolled fashion? We have already seen that cancer cells often produce excessive amounts (or hyperactive versions) of growth factors, receptors, or other components of growth factor signaling pathways.
Such alterations cause an excessive production of the Cdk-cyclins that phosphorylate the Rb protein, thereby providing an ongoing stimulus for cells to pass through the restriction point and divide.
The situation is made even worse by the fact that the restriction point often fails to function properly in cancer cells. When cancer cells are grown under suboptimal conditions—for example, insufficient growth factors, high cell density, lack of anchorage, or inadequate nutrients— that would cause normal cells to become arrested at the restriction point, cancer cells continue to grow and divide without halting at the restriction point.
In other words, cancer cells exhibit a loss of restriction point control. Under extremely adverse conditions, such as severe nutritional deprivation, cancer cells die at random points in the cell cycle rather than arresting at the restriction point.
In addition to the loss of restriction point control, cancer cells frequently exhibit defects in the checkpoint pathways that would otherwise respond to internal problems, such as DNA damage, by halting the cell cycle. Failures in checkpoint pathways, along with the loss of restriction point control, allow cancer cells to continue proliferating under conditions in which the cell cycle of normal cells would stop.
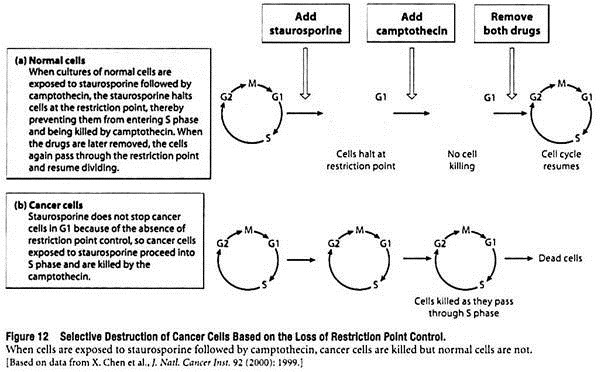
The difference between cell cycle regulation in cancer cells and normal cells can be exploited experimentally using drugs that act at different points in the cycle. For example, staurosporine is a drug that halts the cell cycle at the restriction point, and camptothecin is a drug that kills cells in S phase by disrupting DNA synthesis.
As shown in Figure 12, when cultures of normal cells are exposed to staurosporine followed by camptothecin, the staurosporine halts cells at the restriction point and thus prevents them from entering S phase and being killed by camptothecin. If the two drugs are later removed, the cells again pass through the restriction point and resume dividing.
With cancer cells, the results are quite different. Staurosporine does not stop cancer cells in G1 because of the loss of restriction point control, so the cells proceed into S phase and are killed by camptothecin. This discovery that cancer cells can be killed by drug combinations that do not harm normal cells raises the possibility that similar strategies might eventually be devised for treating cancer patients.
4. Essay on Traits Affecting Cancer Cell Proliferation:
An unrestrained cell cycle, however, is not the only factor that contributes to the uncontrolled production of tumor cells. The number of cells that accumulate in a growing tumor is determined not just by the rate at which cells divide, but also by the rate at which they die. As in the case of the cell cycle, cell death is controlled by pathways that fail to function properly in cancer cells, thereby permitting the survival of cells that would otherwise be destroyed.
Traits that affect cancer cell proliferation by affecting the cell cycle and cell division:
i. Apoptosis is a Mechanism for Eliminating Un-Needed or Defective Cells:
Cell death seems like it would be a random, uncontrolled, undesirable event. In reality, organisms possess a precisely regulated genetic program for inducing individual cells to kill themselves when appropriate. This suicide program, called apoptosis, is designed to prevent the accumulation of unneeded or defective cells that arise during embryonic development as well as later in life.
For example, you might think that embryos would produce only the exact number of cells they need, but that is hardly the case. Embryos produce many extra cells that will not form part of the final organ or tissue in which they arise. A case in point is the human hand, which starts off as a solid mass of tissue.
The fingers are then carved out of the tissue by a process in which apoptosis is invoked to destroy the cells that would otherwise form a webbing between the fingers. Apoptosis is also important in the newly forming brain, where extra nerve cells created during embryonic development are destroyed by apoptosis during early infancy as the final network of nerve connections is established.
Another function of apoptosis is to rid the body of defective cells. For example, cells infected with viruses often invoke apoptosis to trigger their own destruction, thereby limiting reproduction and spread of the virus. Cells with damaged DNA may also trigger apoptosis, especially if the damage cannot be repaired. This ability to destroy genetically damaged cells is especially useful in helping avert the development of cancer.
ii. Apoptosis is Carried Out by a Caspase Cascade:
Apoptosis is a unique type of cell death, quite different from what happens when cells are destroyed by physical injury or exposure to certain poisons. In response to such nonspecific damage, cells undergo necrosis, a slow type of death in which cells swell and eventually burst, spewing their contents into the surrounding tissue. Necrosis often results in an inflammatory reaction that can cause further cell destruction, which makes it potentially dangerous.
In contrast, apoptosis kills cells in a quick and neat fashion without causing damage to surrounding tissue. The process involves a carefully orchestrated sequence of intracellular events that systematically dismantle the cell (Figure 13). The first observable change in a cell undergoing apoptosis is cell shrinkage. Next, small bubble-like protrusions of cytoplasm (“blebs”) start forming at the cell surface as the nucleus and other cellular structures begin to disintegrate.
The chromosomal DNA is then degraded into small pieces and the entire cell breaks apart, forming small fragments known as apoptotic bodies. Finally, the apoptotic bodies are swallowed up by neighboring cells called phagocytes, which are specialized for ingesting foreign matter and breaking it down into molecules that can be recycled for other purposes.
Apoptosis is carried out by a series of protein- degrading enzymes known as caspases. Normally, caspases reside in cells in the form of inactive precursors called procaspases. When a cell receives a signal to commit suicide, an initiating member of the procaspase family is converted into an active caspase.
The activated caspase catalyzes the conversion of another procaspase into an active caspase, which activates yet another procaspase, and so forth. Some members of this caspase cascade destroy key cellular proteins.
For example, one caspase degrades a protein involved in maintaining the structural integrity of the nucleus, and another caspase degrades a protein whose destruction releases an enzyme that causes fragmentation of chromosomal DNA. Hence, the net effect of the caspase cascade is the activation of a series of enzymes that degrade the cell’s main components, thereby leading to an orderly disassembly of the dying cell.
iii. Cancer Cells are able to Evade Apoptosis:
The presence of procaspases within a cell means that the cell is programmed with the seeds of its own destruction, ready to commit suicide quickly if so required. It is therefore crucial that the mechanisms employed to control caspase activation are precisely and carefully regulated and are called into play only when there is a legitimate need to destroy an unneeded or defective cell. There are two main routes for activating the caspase cascade, an external pathway and internal pathway (see Figure 13, bottom).
The external pathway is employed when a cell has been targeted for destruction by other cells in the surrounding tissue. In such cases, neighboring cells produce molecules that transmit a “death signal” by binding to death receptors present on the outer surface of the targeted cell.
The activated death receptors then interact with, and trigger activation of, initiator procaspase molecules located inside the cell, thereby starting the caspase cascade.
The internal pathway—a pathway that is particularly relevant to the field of cancer biology—functions mainly in the destruction of cells that have sustained extensive DNA damage. Although cells possess several mechanisms for repairing DNA damage, in many cases it is safer to destroy cells in which there is any question about the integrity of their DNA.
In this way, the potential danger posed by the proliferation of mutant cells is minimized. The protein plays a pivotal role in the mechanism by which, apoptosis is induced in cells that have sustained extensive DNA damage. The presence of damaged DNA triggers the accumulation of the p53 protein, which in turn stimulates the production of proteins that alter the permeability of mitochondrial membranes. The altered mitochondria then release a group of proteins, especially cytochrome c, that activate the caspase cascade and thereby cause the cell to be destroyed by apoptosis.
Given that killing defective cells is one of the main functions of apoptosis, why aren’t cancer cells destroyed?
After all, cancer cells fit the definition of defective cells- They grow in an uncontrolled fashion and, as you will learn shortly, possess DNA mutations and other chromosomal abnormalities. The reason cancer cells are still able to survive is that they have developed ways of avoiding apoptosis. One common mechanism is that many cancer cells have mutations that disable the gene coding for thereby disrupting the main internal pathway for triggering apoptosis.
Mutations the gene are the most common genetic defect observed in human cancers. Other genes involved in apoptosis may also be altered in cancer cells. For example, the gene coding for the Bcl2 protein, a naturally occurring inhibitor of apoptosis, is altered in some cancers in a way that causes too much Bcl2 to be produced, thereby blocking apoptosis.
5. Essay on the Role of DNA in Cancer Cell Proliferation:
i. Cancer Cells are Genetically Unstable and often Exhibit Gross Chromosomal Abnormalities:
The number of mutations accumulated by cancer cells is generally greater than would be expected in comparable populations of normal cells. This tendency to accumulate an excessive number of mutations and other kinds of DNA damage is called genetic instability.
As we have just seen, one reason for genetic instability is that cancer cells may exhibit defects in DNA repair that diminish their ability to correct DNA mutations. The net result is elevated mutation rates that can be hundreds or even thousands of times higher than normal.
Another factor is that cancer cells often exhibit defects in the pathways that trigger apoptosis in response to DNA damage. As a result, cells that have incurred extensive DNA damage that is beyond repair do not self-destruct by apoptosis as would normally be expected.
Mistakes in the handling of chromosomes during cell division also contribute to genetic instability by creating gross abnormalities in chromosome structure and number. Normally, human cells other than sperm and eggs possess 23 pairs of chromosomes, or a total of 46 chromosomes per cell.
Such cells are said to be diploid (from the Greek word diplous, meaning “double”) because two copies of each type of chromosome are present, one derived from each parent. In contrast, cancer cells are often aneuploid, which means that they possess an abnormal number of chromosomes. Aneuploidy usually involves both the loss of some chromosomes and extra copies of others.
In addition to an abnormal number of chromosomes, cancer cells often possess chromosomes whose structure has been altered by deletions (loss of long stretches of DNA) and translocations (exchange of long stretches of DNA between different chromosomes).
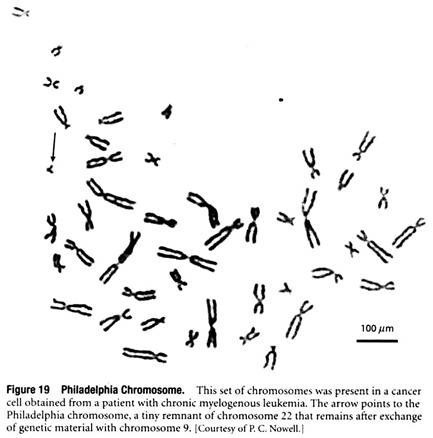
One of the first chromosomal abnormalities to be consistently observed in any type of cancer was the Philadelphia chromosome, an oddly shaped chromosome present in the cancer cells of nearly 90% of all individuals with chronic myelogenous leukemia (Figure 19).
The Philadelphia chromosome is produced by DNA breakage near the ends of chromosomes 9 and 22, followed by reciprocal exchange of DNA between the two chromosomes. A similar phenomenon is observed in Burkitt’s lymphoma, a cancer of human lymphocytes, in which segments derived from chromosomes 8 and 14 are exchanged. In both of these situations, scientists have identified the specific gene whose alteration by chromosomal translocation leads to cancer.
Abnormalities in chromosome number and structure contribute to cancer development in various ways. Some crucial genes may be lost, other genes may become overactive, and yet other genes may become structurally altered and produce abnormal products. In contrast to mutations involving short stretches of DNA, gross chromosomal defects are extremely difficult, if not impossible, to repair.
The best solution for cells exhibiting such chromosomal problems is suicide by apoptosis. However, if cells have genetic defects that disrupt the pathways responsible for carrying out apoptosis, self- inflicted suicide is not an option and the genetically damaged cells will continue to proliferate.
ii. DNA Mutations can Lead to Cancer:
The presence of gross chromosomal defects in cancer cells was first reported almost a hundred years ago, and the smaller changes in base sequence that occur in the DNA of cancer cells have also been recognized for many years.
For a long time, it was difficult to distinguish whether such DNA abnormalities are responsible for causing cancer or whether they simply represent secondary changes that arise because cancer cells are dividing in a rapid, uncontrolled fashion. In other words, the question was much like the classic problem of “which came first, the chicken or the egg?” Do DNA abnormalities cause cancer to arise, or does cancer cause DNA abnormalities to arise?
This issue was finally resolved in the early 1980s, when DNAs isolated from several different human cancers were shown to cause cancer under laboratory conditions. In the first studies of this type (Figure 20), DNA was extracted from human bladder cancer tissue and applied to a culture of normal mouse cells under experimental conditions that favor incorporation of the foreign DNA into the cells’ chromosomes.
The uptake of foreign DNA by cells under such artificial laboratory conditions is called transfection. In response, some of the cultured mouse cells began to proliferate excessively. When these proliferating cells were injected back into mice, the animals developed cancer.
Similar experiments using DNA extracted from normal human tissues did not produce mouse cells capable of forming tumors. It was therefore concluded that DNA obtained from human bladder cancer contains gene sequences, not present in normal DNA, that are capable of causing cancer.
Similar results were subsequently obtained using DNA isolated from other human cancers. Such studies have facilitated the identification of a number of specific genes that contribute to cancer development.
6. Essay on Immune System and Cancer Cell Proliferation:
The ability of cancer cells to proliferate in an uncontrolled fashion, combined with their capacity to spread through the body, makes them a potentially lethal hazard. Are any of the body’s normal defense mechanisms capable of protecting against such a threat? The immune system is designed to defend against infection by potentially harmful agents, such as bacteria, viruses, fungi, parasites, and it also attacks foreign tissues and cells.
Is the immune system also capable of recognizing cancer cells, and if so, how does cancer cells frequently manage to thrive despite the immune system? In addressing these questions, let us start by reviewing the basic mechanisms involved in an immune response.
i. Immune Responses are Carried Out by B Lymphocytes, T Lymphocytes, and NK Cells:
Molecules capable of provoking an immune response are referred to as antigens. To function as an antigen, a substance must be recognized as being “foreign”—that is, different from molecules normally found in a person’s body. The more a molecule differs in structure from normal tissue constituents, the greater the intensity of the immune response mounted against that substance.
Antigens also need to be susceptible to degradation and processing by cells. This requirement explains why non-degradable foreign materials, such as stainless steel pins and plastic valves, can be surgically implanted into humans without eliciting an immune response. To trigger an efficient immune response, an antigen must be degraded and processed by specialized antigen-presenting cells that “present” antigens to cells of the immune system in a way designed to activate an immune response.
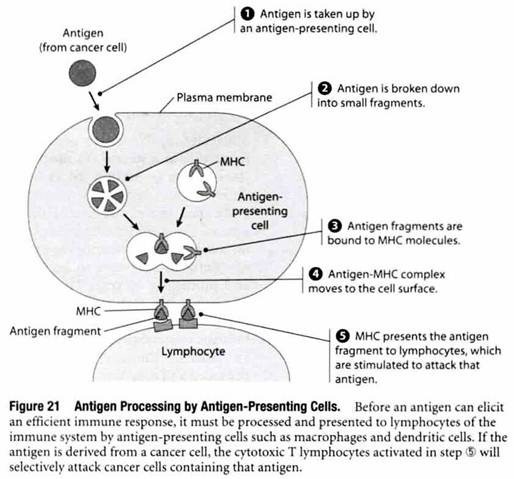
Macrophages and dendritic cells are among the most commonly encountered antigen-presenting cells. As shown in Figure 21, antigens engulfed by these cells are degraded into small fragments that eventually become bound to cell surface proteins called major histocompatibility complex (MHC) molecules. When an antigen fragment bound to an MHC molecule is present at the surface of an antigen- presenting cell, the MHC-antigen complex stimulates cells called lymphocytes to mount an attack against that particular antigen.
The stimulated lymphocytes attack foreign antigens in two different ways. One group of lymphocytes, called B lymphocytes, produce proteins called antibodies, which circulate in the bloodstream and penetrate into extracellular fluids, where they bind to the foreign antigen that induced the immune response. Another group of lymphocytes, called cytotoxic T lymphocytes (CTLs), bind to cells exhibiting foreign antigens on their surface and kill the targeted cells by causing them to burst.
In addition to B and T lymphocytes, a small fraction of the total lymphocyte population consists of natural killer (NK) cells that possess the intrinsic ability to recognize and kill certain kinds of tumor cells (as well as virus-infected cells).
In contrast to a typical immune response involving antibody formation or cytotoxic T lymphocytes, NK cells do not need to recognize a specific antigen before attacking a target cell. Instead, they are programmed to attack a broad spectrum of abnormal cells in a relatively indiscriminate fashion, while leaving normal cells unharmed.
ii. Some Cancer Cells Possess Antigens that Trigger an Immune Response:
Because so many people develop cancer, it is clear that the ability of NK cells to attack tumors is often overwhelmed.
A more powerful and efficient immune response requires the participation of cytotoxic T lymphocytes, whose killing activity is selectively directed at cells containing a specific antigen. For cytotoxic T lymphocytes to become involved, however, they need to recognize an antigen as being foreign or abnormal.
The question of whether cancer cells exhibit unique antigens that can elicit such an immune response has had a long and controversial history. Part of the difficulty in reaching a consensus arises because cancers exhibit a variety of antigenic changes. In human melanomas, for example, at least three classes of antigens have been detected.
Antigens of the first type are specific both for melanomas and for the person from whom a particular melanoma is obtained. Antigens of the second type are specific for melanomas but not for the particular person who has the melanoma. Antigens of the two preceding types cannot be detected in normal cells and are therefore examples of tumor-specific antigens.
Antigens of the third type are present in both normal and melanoma cells, although their concentration in melanoma cells is greater. Such antigens which are present in higher concentration in a tumor but are not unique to tumors are more accurately referred to as tumor-associated antigens.
The distinction between tumor-specific and tumor- associated antigens is sometimes difficult to make. For example, a group of molecules called MAGE antigens are expressed in melanomas and several other cancers but not in most normal tissues. The MAGE antigens are therefore close to being “tumor-specific”, and an immune response directed against them would be expected to be reasonably selective, causing minimum damage to normal tissues.
Other tumor-associated antigens, such as the prostate-specific antigen (PSA) produced by prostate cancer cells, are unique to a specific tissue. Although antigens of this type are produced by normal cells as well, an immune response directed against them would be relatively selective in that it is only directed against a single tissue.
Antigens that are genuinely tumor-specific occur in cancers that produce structurally abnormal proteins. Such proteins do not appear in normal cells and thus can be recognized by the immune system as being “foreign”. There are several examples of mutant cancer cell genes that produce abnormal proteins.
These molecules can act as antigens that elicit a highly selective, cytotoxic T cell response against the tumor cells in which they are found, provided that the mutant proteins are processed and presented to the immune system in the appropriate fashion.
iii. The Immune Surveillance Theory Postulates that the Immune System is able to Protect against Cancer:
The existence of tumor-specific antigens raises an interesting question- Why don’t people with cancer reject their own tumors? The immune surveillance theory postulates that immune destruction of newly forming cancer cells is in fact a routine event in healthy individuals, and cancer simply reflects the occasional failure of an adequate immune response to be mounted against aberrant cells.
The validity of this theory has been debated for many years, with various kinds of evidence being cited both for and against it.
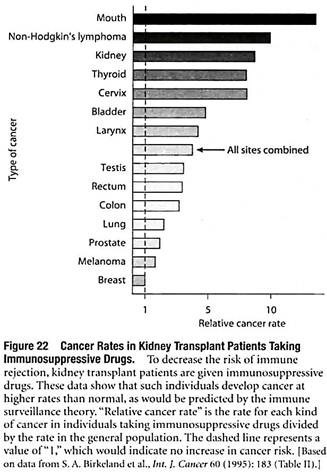
Some of the evidence involves organ transplant patients who take immunosuppressive drugs, which depress immune function and thereby decrease the risk of immune rejection of the transplanted organ. As would be predicted by the immune surveillance theory, individuals treated with immunosuppressive drugs develop many cancers at higher rates than normal (Figure 22).
Although this finding appears to support the idea that the immune system normally helps prevent cancers from developing, it is also possible that the immunosuppressive drugs are acting directly to trigger the development of cancer. For example, cyclosporin—one of the most effective and commonly used immunosuppressive drugs—has been shown to stimulate the proliferation and motility of isolated cancer cells growing in culture.
These results indicate that direct effects of immunosuppressive drugs on newly forming cancer cells may contribute to the increased tumor growth observed in individuals taking such drugs.
A more direct approach for evaluating the immune surveillance theory involves the use of animals that have been genetically altered to introduce specific defects in the immune system. One study of this type employed mutant mice containing disruptions in Rag2, a gene expressed only in lymphocytes.
The mutant mice, which produce no functional lymphocytes, were found to develop cancer more frequently than do normal mice. An increased cancer risk was observed both for cancers that arise spontaneously and for cancers that were induced by injecting animals with a cancer-causing chemical. Such results indicate that a normally functioning immune system helps protect mice against the development of cancer.
Nonetheless, the question still exists as to the relevance of these findings to human cancers. If the immune system plays a significant role in protecting humans from common cancers, you would expect to see a dramatic increase in overall cancer rates in AIDS patients with severely depressed immune function.
While people with AIDS do exhibit higher rates for a few types of cancer, especially Kaposi’s sarcoma and lymphomas, increased rates for the more common forms of cancer have not been observed. Most of the cancers that do occur in higher rates in AIDS patients are known to be caused by viruses.
Such observations suggest that immune surveillance may play an important role in protecting humans from virally induced cancers but that it is less effective in preventing the more common forms of cancer.
iv. Cancer Cells have Various Ways of Evading the Immune System:
Based on the large number of people who develop cancer each year, it is clear that tumors routinely find ways of evading destruction by the immune system. One mechanism is based on tumor progression, which refers to the gradual changes in the makeup of cancer cell populations that occur over time as natural selection favors the survival of cells that are more aggressive and aberrant.
During tumor progression, cells containing antigens that elicit a strong immune response are most likely to be attacked and destroyed. Conversely, cells that either lack or produce smaller quantities of antigens marking them for destruction are more likely to survive and proliferate. So as tumor progression proceeds, there is a continual selection for cells that invoke less of an immune response.
Cancer cells have also devised ways of actively confronting and overcoming the immune system. For example, some cancer cells produce molecules that kill T lymphocytes or disrupt their ability to function.
Tumors may also surround themselves with a dense layer of supporting tissue that shields them from immune attack. And some cancer cells simply divide so quickly that the immune system cannot destroy them fast enough to keep tumor growth in check. Consequently, the larger a tumor grows, the easier it becomes to overwhelm the immune system.
Although tumors are often successful at evading immune attack, immune rejection is not necessarily an unattainable objective. Experiments in mice have shown that immunizing an animal with tumor antigens can trigger an effective immune response under conditions in which the tumor growing in the animal had not elicited any response on its own. Observations like this one suggest that it may be possible to stop or even prevent the development of cancer by stimulating a person’s immune system to attack cancer cells.
7. Essay on Molecular Changes in Cancer Cell Proliferation:
Some of these alterations involve unique or abnormal proteins that are not ordinarily encountered in adult tissues, but most of the molecular changes seen in cancer cells proliferation are increases or decreases in the quantity or activity of normal proteins. We will examine a few such changes that are especially noteworthy.
i. Cancer Cells Exhibit Cell Surface Alterations that Affect Adhesiveness and Cell-Cell Communication:
Alterations in the makeup of the outer cell membrane (plasma membrane) are almost universally observed in cancer cells, yet it is often difficult to assess the significance of such changes because similar alterations occur in normal cells when they begin to proliferate.
For example, membrane transport proteins responsible for the uptake of sugars, amino acids, and other nutrients are frequently activated in tumor cells, but similar increases are observed in normal cells that have been stimulated to divide by adding nutrients or growth factors. It is therefore important to distinguish between membrane changes that play unique roles in cancer cells and membrane changes that are characteristic of dividing cells in general.
Among the cell surface changes that are particularly distinctive and important for the behavior of cancer cells are those that influence adhesiveness. In normal tissues, cell-cell adhesion helps keep cells in their place; in cancers, this adhesiveness is diminished or missing entirely. The reduced adhesiveness of cancer cells can often be traced to defects in E-cadherin, a cell-cell adhesion protein that is located at the cell surface.
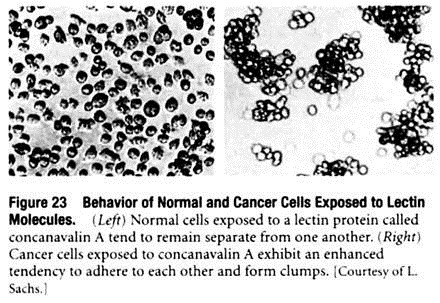
A second cell surface property altered in cancer cells is their enhanced tendency to clump together when exposed in the laboratory to proteins called lectins. A lectin is a carbohydrate-binding protein possessing two or more carbohydrate-binding sites, which means that a single lectin molecule can link two cells together by binding to carbohydrate groups exposed on the surface of each cell.
As a result, when lectins are added to a suspension of isolated cells, the lectin molecules link the cells together to form large clumps (Figure 23). It was initially thought that the increased susceptibility to lectin-induced clumping meant that cancer cells possess more cell surface carbohydrate groups to which lectins can bind.
Careful measurements, however, have led to the conclusion that the total number of these carbohydrate groups tends to be similar in normal and cancer cells. What does appear to differ is the mobility of carbohydrate groups within the plasma membrane, which tends to be greater in cancer cells than in normal cells. This ability of cell surface carbohydrate groups to move more readily in cancer cells apparently increases the rate at which they can bind to added lectins.
Another cell surface alteration commonly observed in cancer cells is a decrease in the number of gap junctions, which are specialized cell surface structures composed of a protein called connexin. Gap junctions play a role in cell- cell communication by joining adjacent cells together in a way that allows small molecules to pass directly from one cell to another.
The idea that gap junctions are deficient in cancer cells has come from experiments showing that small fluorescent molecules injected into normal cells move rapidly into surrounding cells that are normal but not into cells that are malignant (Figure 24). To investigate whether this deficiency plays any role in the loss of growth control, studies have been performed in which normal cells were fused with cancer cells that had lost the ability to form gap junctions.
Initially, the resulting hybrid cells produced gap junctions and exhibited normal growth control, but the gap junctions eventually disappeared from some of the hybrid cells. At that point the cells reverted to uncontrolled growth; raising the interesting possibility that normal growth control is influenced by the ability of cells to communicate through gap junctions.
ii. Cancer Cells Produce Embryonic Proteins, Proteases, and Stimulators of Blood Vessel Growth:
A great deal of effort has been expended in searching for molecules that are produced only by cancer cells and that might therefore serve as “markers” for detecting the presence of cancer. Unfortunately, few of the molecules identified thus far are broadly useful as unique identifiers of cancer cells, although a number of them can be employed for detecting the presence of specific kinds of cancer. For example, some cancer cells manufacture and secrete proteins that are usually found only in embryos.
One such protein, alpha-fetoprotein, is produced by embryonic liver cells but is detectable in only trace amounts in normal adults. In people with liver cancer, the concentration of alpha-fetoprotein in the blood increases dramatically. Carcinoembryonic antigen (CEA), a protein produced in the embryonic digestive tract, and fetal hormones such as chorionic gonadotropin and placental lactogen, are also secreted by some cancers.
Blood tests for embryonic markers such as alpha-fetoprotein and carcinoembryonic antigen can therefore be used to monitor the presence of certain kinds of cancer, but the fact that these substances are made by only a few tumor types limits the applicability of this approach.
Other proteins produced by cancer cells are not unique to such cells but have provided some important insights into the behavior of malignant tumors. For example, cancer cells tend to produce proteases (protein- degrading enzymes) that facilitate the breakdown of structures that would otherwise represent barriers to cancer cell movement and invasion.
Although proteases are also secreted by certain kinds of normal cells, their enhanced production by cancer cells facilitates the ability of malignant tumors to invade surrounding tissues and enter the circulatory system. Cancer cells also produce proteins that stimulate the growth of blood vessels, thereby helping ensure that tumors have a sufficient blood supply.