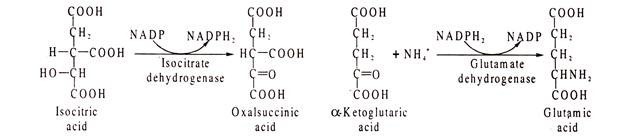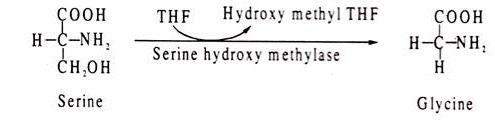ADVERTISEMENTS:
In this article we will discuss about the structure and function of various coenzymes.
1. NAD/NADP:
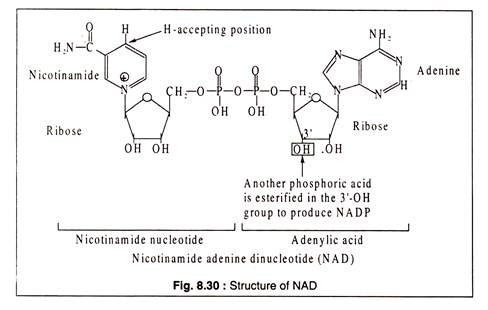
Nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) are derivatives of the B-vitamin, nicotinic acid.
The structures are shown in Fig. 8.30:
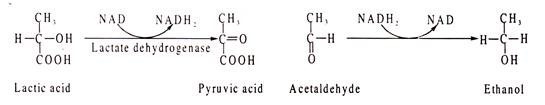
NAD and NADP act as conezymes for many degydrogenases where they are involved in transfer of hydrogen, causing either oxidation or reduction of the substrates. In general, NAD takes part in the catabolic reactions, which NADP in synthetic pathway reactions. Some NAD containing dehydrogenases are lactic dehydrogenase, alcohol dehydrogenase, malate dehydrogenase, glycerin aldehyde phosphate dehydrogenase etc.
Two examples are cited below, one of reduction and the other or oxidation:
Lactic acid is oxidized to pyruvic acid where NAD acts as H-acceptor. In the other reaction, acetaldehyde is reduced to ethanol where NADH2 acts as H-donor.
ADVERTISEMENTS:
Example of NADP catalysed reacted are glucose 6-phosphate dehydrogenase, isocitrate dehydrogenase, glutamic acid dehydrogenase etc.:
Although the reduced forms of NAD and NADP are usually shown as NADH2 and NADPH2 for convenience, it should be noted that the correct forms should be NADH+H+ and NADPH+H+ respectively, because the positively charged nicotinamide ring accepts one electron and one H-atom from a pair of H-atoms removed from the substrate. The electron goes to the positively charged N-atom and another hydrogen is added at the position shown in Fig. 8.30.
NAD and NADP were previously called DPN (diphosphopyridine nucleotide) and TPN (triphospho pyridine nucleotide), respectively.
2. Flavin Mononucleotide (FMN) and Flavin Adenine Dinucleotide (FAD):
FMN and FAD, commonly called flavoproteins, are also hydrogen transferring coenzymes associated with hydrogenases. The coenzyme parts of these flavoproteins contain the B-vitamin, riboflavin. In contrast to NAD or NADP, the coenzymes of flavoproteins are more tightly bound to the apoenzyme. As a result they cannot be separated by dialysis.
The structures of riboflavin, FMN and FAD are shown in Fig. 8.31:
On reduction of FAD by addition of two H-atoms donated by a substrate, it is converted to FADH2. The H-accepting positions are shown in Fig. 8.32. The substrate is thereby oxidized. An example of FAD containing enzyme is succinate dehydrogenase occurring in the Krebs’ cycle. Succinic acid is oxidized to fumaric acid by the enzyme. The hydrogen accepted by FAD is transferred to the electron transport chain for generation of ATP.
3. Coenzyme A (CoA):
Coenzyme A has a complex structure consisting of an adenosine triphosphate, a pantothenic acid which is a B-vitamin and cysteamine. The coenzyme is involved in transfer of acyl-groups. The sulfhydryl (-SH) group of cysteamine moiety of this coenzyme forms a thioester with the carboxyl (-COOH) group of the acyl-compound, such as acetic acid to produce acetyl-CoA which is one of the most important CoA derivatives. The thioester bond is energy-rich and can easily transfer the acetyl- group to an acceptor.
The structure of coenzyme A, formation of a thioester and a reaction involving coenzyme A are shown in Fig. 8.33:
4. Thiamine Pyrophosphate (TPP):
TPP is a coenzyme involved in transfer of aldehyde (—C—H) groups, like acetaldehyde and glycol aldehyde. It contains thiamine, a vitamin of B-group. The thiazole group of the coenzyme molecule accepts the aldehyde group and transfers it to an acceptor via other coenzymes, like lipoic acid and coenzyme A. TPP is involved in oxidative decarboxylation of pyruvic acid and α-ketoglutaric acid.
ADVERTISEMENTS:
The structures of TPP and ‘active’ acetaldehyde are shown in Fig. 8.34:
An example of an enzyme complex involving TPP, lipoic acid and coenzyme A is the pyruvate decarboxylase.
ADVERTISEMENTS:
The reaction is shown in a simplified way (Fig. 8.35):
5. Pyridoxal Phosphate (PAL):
Pyridoxal phosphate is a coenzyme associated with — transaminases which catalyse transfer of amino groups from amino acids to keto acids. In this transfer process, PAL acts as the acceptor of the amino group and is converted to pyridoxamine phosphate (PAM).
PAM can react with a keto acid to produce an amino acid. PAL and PAM remain bound to the protein part of the transaminase enzyme during these transfer of amino group. The reactions catalysed by transaminases can be represented in a simple way as shown in Fig. 8.36. Pyridoxal phosphate has a simple molecule containing the B-vitamin, pyridoxine.
ADVERTISEMENTS:
The structures of PAL and PAM are shown in Fig. 8.36:
The aldehyde group of PAL is the reactive group of the coenzyme which binds to the amino acid forming a Schiff s base.
The details of transaminase reaction are shown in Fig. 8.37:
6. Other Molecules having Coenzyme Function:
These include lipoic acid (thioctic acid), biotin, tetrahydrofolic acid and cobalamine.
ADVERTISEMENTS:
The structures of some of these compounds are shown in Fig. 8.38:
Lipoic acid is involved in oxidative decarboxylation reactions, such as those catalysed by pyruvic decarboxylase or α-keto glutarate decarboxylase. Biotin is bound to enzymes involved in carboxylation reactions. In such reactions biotin acts as the carrier of CO2. The CO2-biotin compound is known as active CO2. An example is pyruvate carboxylase which adds a CO2 molecule to pyruvic acid forming oxalacetic acid.
Tetrahydrofolic acid (THF) acts as coenzyme for enzymes involved in transfer of one-carbon fragments, like formyl, methyl and methenyl groups. An example of a reaction involving THF is conversion of homocysteine to methionine. The methyl group of methionine is added from methyl-THF. Another THF mediated reaction is conversion of serine to glycine where the hydroxy-methyl group of serine is removed by THF.
Cobalamine or vitamin B12 is a cobalt-containing complex molecule composed of 63 carbon atoms, a tetrapyrole ring system and a nucleotide. The cobalt atom is held in the tetrapyrole ring and carries a cyano (-CN) group. Cobalamine acts as coenzyme for enzymes catalyzing intra-molecular transfer of carboxyl group. An important reaction of this type is conversion of methyl malonyl- coenzyme A to succinyl-coenzyme A.