ADVERTISEMENTS:
This article throws light upon the top five types of methods devised for enzyme kinetics measurement.
The top five types of methods are: (1) Detection Methods (2) Assays Based on Optical Spectroscopy (3) Fluorescence Measurements (4) Radio Isotopic Measurements and (5) Other Detection Methods.
Method # 1. Detection Methods:
A wide variety of physicochemical methods have been used to follow the course of enzymatic reactions.
ADVERTISEMENTS:
Some of the more common techniques are described here, but our discussion is far from comprehension. Any signal that differentiates the substrates or products of the reaction from the other components of the reaction mixture can, in principle, form the basis of an enzyme assay; the only limit is the creativity of the investigator.
Method # 2. Assays Based on Optical Spectroscopy:
Two very common means of following the course of an enzymatic reaction are absorption spectroscopy and fluorescence spectroscopy. Both methods are based on the changes in electronic configuration of molecules that result from their absorption of light energy of specific wavelengths. Molecules can absorb electromagnetic radiation, such as light, causing transitions between various energy levels.
Energy in the infrared region, for example, can cause transitions between vibrational levels of a molecule. Microwave energy can induce transitions among rotational energy levels, while radiofrequency energy, which forms the basis of NMR spectroscopy, can cause transitions among nuclear spin levels. The energy differences between electronic levels of a molecule are so large that light energy in the ultraviolet (UV) and visible regions of the electromagnetic spectrum is required to induce transitions among these states.
ADVERTISEMENTS:
If a molecule is irradiated with varying wavelengths of light (of similar intensity), only light of specific wavelengths will be strongly absorbed by the sample. At these wavelengths, the energy of the light matches the energy gap between two electronic states of the molecule, and the light is absorbed to induce such a transition. Fig. 7.6 is a hypothetical absorption spectrum of a molecule in which light of wavelength lmax induces an electron redistribution to bring the molecule from its ground π state to an excited π* state. This process is illustrated as a potential energy diagram in Fig. 7.7A.
Absorption Measurements:
The value of absorption spectroscopy as an analytical tool is that the absorption of a molecule at a particular wavelength can be related to the concentration of that molecule in solution, as described by Beer’s law:
A = ɛcl,
where A is the absorbance of the sample at some wavelength, c is the concentration of sample in molarity units, 1 is the path length of sample the light beam traverses (in centimeters), and ɛ is an intrinsic constant of the molecule, known as the extinction coefficient, or molar absorptivity.
Since absorbance is a unit-less quantity, ɛ must have units of reciprocal molarity time’s reciprocal path length (typically expressed as M-1 cm-1 or mM-1 cm-1). Thus, if we know the value of ɛ for a particular molecule, and the path length of the cuvette, we can calculate the concentration of that molecule in a solution by measuring the absorption of that solution. As we have seen in the examples of Figs. 7.1 and 7.5, we can use the unique absorption features of a substrate or product of an enzymatic reaction to follow the course of such a reaction.
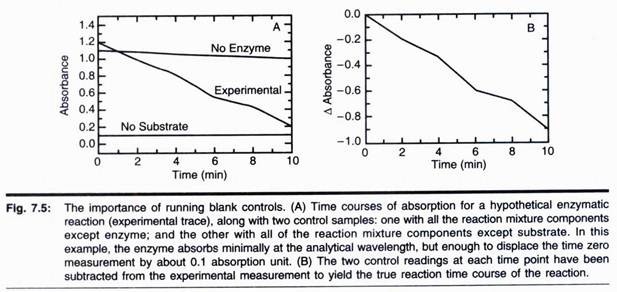 Using Beer’s law, we can convert our measured AA values at different time points to the concentration of the molecule being followed, and thus report the reaction velocity in terms of change in molecular concentration as a function of time. Note that the units here, molarity per unit time (i.e., moles per litre per unit time) are commonly used in reporting enzyme velocities. Some workers instead report velocity in units of moles per unit time. The two units are easily interconverted by making note of the total volume of the reaction mixture for which the velocity is being measured.
Using Beer’s law, we can convert our measured AA values at different time points to the concentration of the molecule being followed, and thus report the reaction velocity in terms of change in molecular concentration as a function of time. Note that the units here, molarity per unit time (i.e., moles per litre per unit time) are commonly used in reporting enzyme velocities. Some workers instead report velocity in units of moles per unit time. The two units are easily interconverted by making note of the total volume of the reaction mixture for which the velocity is being measured.
Choosing an Analytical Wavelength:
The wavelength used for following enzyme kinetics should be one that gives the greatest difference in absorption between the substrate and product molecules of the reaction. In many cases, this will correspond to the wavelength maximum of the substrate or product molecule. However, when there is significant spectral overlap between the absorption bands of the substrate and product, the most sensitive analytical wavelength may not be the same as the wavelength maximum.
ADVERTISEMENTS:
This concept is illustrated in Fig. 7.8. In inspecting the spectra for the substrate and product of the hypothetical enzymatic reaction of Fig. 7.8A, note that at the wavelength maximum for each molecule, the other molecule displays significant absorption. The wavelength at which the largest difference in signal is observed can be determined by calculating the difference spectrum between these two spectra, as illustrated in Fig. 7.8B.
Thus, in our hypothetical example, the wavelength maxima for the substrate and product are 374 nm and 385 nm, respectively, but the most sensitive wavelengths for following loss of substrate or formation of product would be 362 nm and 402 nm, respectively.
Optical Cells:
Absorption measurements are most commonly performed with a standard spectrophotometer, and the samples are contained in specialized cells known as cuvettes. These specialized cells come in a range of path lengths and are constructed of various optical materials. Disposable plastic cuvettes that hold 1 ml or 3 ml samples are commercially available.
ADVERTISEMENTS:
Although these disposable units are very convenient and reduce the chances of sample-to-sample cross-contamination, their use should be restricted to the visible wavelength range (350-800 nm). For measurements at wavelengths less than 350 nm, high quality quartz cuvettes must be used, since both plastic and glass absorb too much light in the ultraviolet.
Quartz cuvettes are available from numerous manufacturers in a variety of sizes and configurations. Regardless of the type of cuvette selected, its path length must be known to ensure correct application of Beer’s law to the measurements.
Usually, the manufacturer provides this information at the time of purchase. If, for any reason, the path length of a particular cuvette is not known with certainty, it can be determined experimentally by measuring the absorption of any stable chromophoric solution in a standard 1 cm path length cuvette and then measuring the absorption of the same sample in the cuvette of unknown path length.
The ratio of the two absorption readings (Aunknown/A1 cm) yields the path length of the unknown cuvette in centimeters. A good standard solution for this purpose can be prepared as follows (Haupt, 1952). Weigh out 0.0400 g of potassium chromate and 3.2000 g of potassium hydroxide and dissolve both in 700 ml of distilled water. Transfer to a one-litre volumetric flask and bring the total volume to 1000 ml with additional distilled water. Mix the solution well. The resulting solution will have absorption of 0.991 at 375 nm in a 1 cm cuvette.
ADVERTISEMENTS:
Many workers now perform absorption measurements with 96-well microtiter plate readers. These devices have remarkable sensitivity (many can measure changes in absorption of as little as 0.001) and can greatly increase productivity by allowing up to 96 samples to be assayed simultaneously. Most plate readers are based on optical filters rather than monochromators, hence providing only a finite number of analytical wavelengths for measurements.
It is usually not difficult, however, to purchase an optical filter for a particular wavelength and to install it in the plate reader. Because most commercially available microtiter plates are constructed of plastic, plate reader measurements are normally restricted to the visible region of the spectrum.
Recently, however, monochromator-based plate readers have come on the market that allows one to make measurements at any wavelength between 250-750 nm. The manufacturers of these instruments also provide special quartz-bottomed plates for performing measurements in the ultraviolet.
As with nonstandard cuvettes, it is necessary to determine the path length through which one is making measurements in a microtiter plate well. The path length in this situation will depend on the total volume of reaction mixture in the well. For example, a 200 µl solution in a well of a 96-well plate has an approximate path length of 0.7 cm. The exact path length under given experimental conditions should be determined empirically, as described above.
ADVERTISEMENTS:
Filter-based plate readers may show an apparent extinction coefficient of a molecule that differs from corresponding measurements made with a conventional spectrometer. Such discrepancies are due in part to the broader bandwidths of the optical filters used in plate readers.
The absorption readings thus survey a wider range of wavelengths than the corresponding spectrometer measurements. Therefore, it is important to use the plate reader under the same conditions as the experimental measurements to determine a standard curve of absorption as a function of chromophore concentration.
Mixing of samples can be problematic in a 96-well plate, since the inversion method cannot conveniently be applied. Instead, many commercial plate readers have automatic built-in shaking devices that allow for sample mixing. Another way to ensure good mixing is to alter the general method just described as follows. The components of the reaction mixture, except for the initiating reagent, are mixed together in sufficient volume for all the wells of the plate to be used. This solution is placed in a multichannel pipette reservoir.
The initiating solution is added to the individual wells of the plate, and the remainder of the reaction solution is added to the wells by means of a multichannel pipet. The solutions are mixed by repeatedly pulling up and dispensing the reaction mixture with the pipeette. An entire row of 12 wells can be mixed in this way in less than 10 seconds.
Errors in Absorption Spectroscopy:
A common error associated with absorption measurements is deviation from Beer’s law. The form of Beer’s law suggests that the absorption of a sample will increase linearly with the concentration of the molecule being analyzed, and indeed, this is the basis for the use of absorption spectroscopy as an analytical tool.
ADVERTISEMENTS:
Experimentally, however, one finds that this linear relationship holds only over a finite range of absorption values. As illustrated in Fig. 7.9, absorption readings greater than 1.0 in general should not be trusted to accurately reflect the concentration of analyte in solution. Thus, experiments should be designed so that the maximum absorption to be measured is less than 1.0.
With a few preliminary trials, it usually is possible to adjust conditions so that the measurements fall safely below this limit. Additionally, the amount of instrumental noise in a measurement is affected by the overall absorption of the sample. For this reason it is more difficult to measure a small absorption change for a sample of high absorption.
Empirically it turns out that the best compromise between minimizing this noise and having a reasonable signal follow occurs when the sample absorption is in the vicinity of 0.5. This is usually a good target absorption for following small absorption changes.
The lamps used to generate the UV and visible light for absorption spectrometers must be given ample time to warm up. The light intensity from these sources varies considerably shortly after the lamps are turned on, but stabilizes after about 30-90 minutes.
Since the amount of warm up time needed to stabilize the lamp output will vary from instrument to instrument, and from lamp to lamp within the same instrument, it is best to determine the required warm-up time for one’s own instrument. This is easily done by measuring the signal from a sample of low absorption (say, ~0.05-0.1) as a function of time after turning the lamp on, and noting how long it takes for the signal to reach a stable, constant reading.
Another source of error in absorption measurements is sample turbidity. Particulate matter in a solution will scatter light that is detected as increased absorption by the sample. If settling of such particles occurs during kinetic measurements, significant noise in the data may result, and in severe cases there will appear to be an additional kinetic component of the data. The best way to avoid these complications is to ensure that the sample is free of particles by filtering all the solutions through 0.2 µm filters or by centrifugation.
Method # 3. Fluorescence Measurements:
Light of an appropriate wavelength can be absorbed by a molecule to cause an electronic transition from the ground state to some higher lying excited state, as we have discussed. Because of its highly energetic nature, the excited state is short-lived (excited state lifetimes are typically less than 50 ns), and the molecule must find a means of releasing this excess energy to return to the ground state electronic configuration.
ADVERTISEMENTS:
Most of the time this excess energy is released through the dissipation of heat to the surrounding medium. Some molecules, however, can return to the ground state by emitting the excess energy in the form of light. Fluorescence, the most common and easily detected of these emissive processes, involves singlet excited and ground electronic states.
The energetic processes depicted in Figure 7.7 are characteristic of molecular fluorescence. First, light of an appropriate wavelength is absorbed by the molecule, exciting it to a higher lying electronic state (Fig. 7.7A). The molecule then decays through the various high energy vibrational sub-states of the excited electronic state by heat dissipation, finally, relaxing from its lowest vibrational level to the ground electronic state with release of a photon (Fig. 7.7B).
Because of the differences in equilibrium interatomic distances between the ground and excited states, and because of the loss of energy during the decay through the higher energy vibrational sub-states, the emitted photon is far less energetic than the corresponding light energy required exciting the molecule in the first place. For these reasons, the fluorescence maximum of a molecule is always at a longer wavelength (less energy) than the absorption maximum; this difference in wavelength between the absorption and fluorescence maxima of a molecule is referred to as the Stokes shift.
For example, the amino acid tryptophan absorbs light maximally at about 280 nm and fluoresces strongly between 325-350 nm (Copeland, 1994). To take advantage of this behaviour, fluorescence instruments are designed to excite a sample in a cuvette with light at the wavelength of maximal absorption and detect the emitted light at a different (longer) wavelength.
To best detect the emitted light with minimal interference from the excitation light beam, most commercial fluorometers are designed to collect the emitted light at an angle of 90° from the excitation beam path. Thus, unlike cells for absorption spectroscopy, fluorescence cuvettes must have at least two optical quality widows at right angles to one another; all four sides of most fluorescence cuvettes have polished optical surfaces.
The strategies for following enzyme kinetics by fluorescence are similar to those just described for absorption spectroscopy. Many enzyme substrates-product pairs are naturally fluorescent and provide convenient signals with which to follow their loss or production in solution.
If these molecules are not naturally fluorescent, it is often possible to covalently attach a fluorescent group without significantly perturbing the interactions with the enzyme under study. Fluorescence measurements offer two key advantages over absorption measurements for following enzyme kinetics.
First, fluorescence instruments are very sensitive, permitting the detection of much lower concentration changes in substrate or product. Second, since many fluorophores have large Stokes shifts, the fluorescence signal is typically in an isolated region of the spectrum, where interferences from signals due to other reaction mixture components are minimal.
Fluorescence signals track linearly with the concentration of fluorophore in solution over a finite concentration range. In principle, fluorescence signals should vary with fluorophore concentration by a relationship similar to Beer’s law, where the extinction coefficient is replaced by the molar quantum yield (Φ). In practice, however, it is difficult to calculate sample concentrations by means of applying tabulated values of Φ to experimental fluorescence measurement.
This limitation is in part due to the nature of the instrumentation and the measurements. Thus, to convert fluorescence intensity measurements into concentration units, it is necessary to prepare a standard curve of fluorescence signal as a function of fluorophore concentration, using a set of standard solutions for which the fluorophore concentration has been determined independently.
The standard curve data points must be collected at the same time as the experimental measurements; however, since day-to-day variations in lamp intensity and other instrumental factors can greatly affect fluorescence measurements. Sometimes the fluorophore is generated only as a result of the enzymatic reaction, and it is difficult to obtain a standard sample of this molecule for construction of a standard curve. In such cases it may not be possible to report velocity in true concentration units, and units of relative fluorescence per unit time must be used instead.
It is still important to quantify this fluorescence relative to some standard fluorescent molecule, to permit comparisons of relative fluorescence measurements from one day to the next and from one laboratory to another. A good standard for this purpose is quinine sulphate.
A dilute solution of quinine sulphate in an aqueous sulphuric acid solution can be excited at any wavelength between 240-400 nm to yield a strong fluorescence signal that maximizes at 453 nm (Fletcher, 1969).
Russo (1969) suggests the following protocol for preparing a quinine sulphate solution as a standard for fluorescence spectroscopy:
i. Weigh out 5 mg of quinine sulphate di-hydrate and dissolve in 100 ml of 0.1 (N) H2SO4.
ii. Measure the absorption of the sample at 366 nm, and adjust the concentration with 0.1 (N) H2SO4 so that the solution has absorption of 0.40 at this wavelength in a 1 cm cuvette.
iii. Dilute a sample of this solution 1/10 with 0.1 (N) H2SO4 and use the solution to record the fluorescence spectrum.
The relative fluorescence of a sample can then be reported as the fluorescence intensity of the sample at some wavelength, divided by the fluorescence intensity of the quinine sulphate standard at 453 nm, when the same fluorometer is used to excite both sample and standard, at the same wavelength. Of course, both sample and standard measurements must be made under the same set of experimental conditions (monochrometer slit width, lamp voltage, dwell time, etc.), and the second set should be made soon after the first.
Internal Fluorescence Quenching and Energy Transfer:
If a molecule absorbs light at the same wavelength at which another molecule fluoresces, the fluorescence from the second molecule can be absorbed by the first molecule, leading to a diminution or quenching of the observed fluorescence intensity from the second molecule (Note that this is only one of numerous means of quenching fluorescence).
The first molecule may then decay back to its ground state by radiation less decay (e.g., heat dissipation), or it may itself fluoresce at some characteristic wavelength. We refer to the first process as quenching because the net effect is a loss of fluorescence intensity. The second situation is described as “resonance energy transfer”, because here excitation at the absorption maximum of one molecule leads to fluorescence by the other molecule (Fig. 7.10).
Both these processes depend on several factors, including the spatial proximity of the two molecules. This property has been exploited to develop fluorescence assays for proteolytic enzymes based on synthetic peptide substrates. The basic strategy is to incorporate a fluorescent group (the donor into a synthetic peptide on either the N- or C-terminal side of the scissile peptide bond that is recognized by the target enzyme.
A fluorescence quencher or energy acceptor molecule (both referred to hereafter as the acceptor molecule) is also incorporated into the peptide on the other side of the scissile bond. When the peptide is intact, the donor and acceptor molecules are covalently associated and remain apart at a relatively fixed distance, able to energetically interact.
Once hydrolyzed by the enzyme, however, the two halves of the peptide will diffuse away from each other, thus eliminating the possibility of any interaction between the donor and acceptor. The observed effect of this hydrolysis will be an increase in the fluorescence from the donor molecule, and, in the case of energy transfer, a concomitant decrease in the fluorescence of the acceptor molecule with execution under the absorption maximum of the donor.
These approaches have been used to follow hydrolysis of peptide substrates for a large variety of proteases (e.g., Matayoshi et al., 1990; Knight et al., 1992; Knight, 1995; and Packard et al., 1997). Table 7.3 summarizes some donor—acceptor pairs that are commonly used in synthetic peptide substrates for proteases. Another good source for information on donor— acceptor pairs are the Internet site of the Molecular Probes Company, a company specializing in fluorescence tools for biochemical and biological research.
“Dabcyl, 4-(4-dimethylaminophenylazo) benzoic acid; Edans, 5-[(2-aminoethyl) amino] naph- thalene-1-sulphonic acid; Dansyl, (5-dimethylaminonaphthalene-1-sulphonyl); DNP, 2, 4-dinitrophenyl; MCA, 7-methoxycoumarin-4 acetic acid; Abz, o-aminobenzyl; Tyr (NO2), 3-nitrotyrosine.
Recently, fluorescence resonance energy transfer (FRET) has been applied to the study of enzymatic group transfer reactions, and to the study of protein-protein interactions in solution. Space does not permit a review of these applications. The interested reader is referred to the online handbook from the Molecular Probes Company for more information and literature examples of biochemical applications of FRET Technology.
Errors in Fluorescence Measurements:
Most of the caveats described for absorption spectroscopy hold for fluorescence measurements as well. Samples must be free of particulate matter, since light scattering is a severe problem in fluorescence. Many of the commonly used fluorophores emit light in the visible region but must be excited at wavelengths in the near ultraviolet, necessitating the use of quartz cuvettes for these measurements. Also, any fluorescence due to buffer components and soon must be measured and corrected to ensure that meaningful data are obtained.
In addition to these, more common considerations are several sources of error unique to fluorescence measurements. First, many fluorescent molecules are prone to photodecomposition after long exposure to light. Hence, fluorescent substrates and reagents should be stored in amber glass or plastic, and the containers should be wrapped in aluminum foil to minimize exposure to environmental light.
Second, the quantum yield of fluorescence for any molecule is highly dependent on sample temperature. We shall see shorty that temperature affects enzyme kinetics directly, but this is distinct from the general influence of temperature on fluorescence intensity. In general, the fluorescence signal increases with decreasing temperature, as competing non-radiative decay mechanisms for return to the ground state become less efficient. Hence, good temperature control of the sample must be maintained. Most commercial fluorometers provide temperature control by means of jacketed sample holders that attach to circulating water baths.
Finally, a major source of error in fluorescence measurements is light absorption by the sample at high concentrations. Individual molecules in a sample may be excited by the excitation light beam and caused to fluoresce.
To be detected, these emitted photons must traverse the rest of the sample and escape the cuvette to impinge on the surface of the detection device (typically a photomultiplier tube or diode array). Any such photon will be lost from detection; however, if before escaping the sample it encounters another molecule that is capable of absorbing light at that wavelength. As the sample concentration increases, the likelihood of such encounters and instances of light reabsorption increases exponentially.
This phenomenon, referred to as the inner filter effect (Fig. 7.11), can dramatically reduce the fluorescence signal observed from a sample. The inner filter effect can be corrected if the absorption of the sample is known at the excitation and emission wavelengths used in the fluorescence measurement. The true, or corrected fluorescence Fcorr can be calculated from the observed fluorescence Fobs as follows (Lackowicz, 1983):
where Aex and Aem are the sample absorptions at the excitation and emission wavelengths, respectively. This correction works only over a limited sample absorption range. If the sample absorption is greater than about 0.1, the correction will not be adequate. Hence, a good rule of thumb is to begin with samples that have absorption values of about 0.05 at the excitation wavelength. The sample concentration can be adjusted from this starting point to optimize the signal-to-noise ratio, with care taken not to introduce a significant inner filter effect.
Method # 4. Radio Isotopic Measurements:
The basic strategy for the use of radioisotopes in enzyme kinetic measurements is to incorporate into the structure of the substrate a radioactive species that will be retained in the product molecule after catalysis. Using an appropriate technique for separating the substrate from the product, one can then measure the amount of radioactivity in the substrate and product fractions, and thus quantify substrate loss and product production. Most of the isotopes that are used commonly in enzyme kinetic measurements decay through emission of β-particles (Table 7 4).
The decay process follows first-order kinetics, and the loss (or disintegration) of the starting material is thus associated with a characteristic half-life for the parent isotope. The standard unit of radioactivity is the curie (Ci), which originally defined the rate at which 1 gram of radium-226 decays completely. Relating this to other isotopes, a more useful working definition of the curie is that quantity of any substance that decays at a rate of 2.22 × 1012 disintegrations per minute (dpm).
Solutions of p-terphenyl or stilbene, in xylene or toluene, will emit light when in contact with a radioactive solute. This light emission, known as scintillation, is most commonly measured with a scintillation counter, an instrument designed around a photomultiplier tube or other light detector. Radioactivity on flat surfaces, such as thin-layer chromatography (TLC) plates and gels can be measured by scintillation counting after the portion of the surface containing the sample has been scraped or cut out and immersed in scintillation fluid.
Another common means of detecting radioactivity on such surfaces entails placing the surface in contact with a sheet of photographic film. The radioactivity darkens the film, making a permanent record of the location of the radioactive species on the surface. This technique, called autoradiography, is one of the oldest methods known for detecting radioactivity. Today computer interfaced phosphor imaging devices also are commonly used for locating and quantifying radioactivity on two-dimensional surfaces (dried gels, TLC plates, etc.).
Radioactivity in a sample is quantified by measuring the dpm’s of a sample using one of the methods just described. Since, however, no detector is 100% efficient, any instrumental reading obtained experimentally will differ from the true dpm of the sample. The experimental units of radioactivity are referred to as counts per minute (cmp’s: events detected or counted by the instrument per minute).
For example, a 1 µCi sample would display 2.22 × 106 dpm. If the detector used to measure this sample had an efficiency of 50%, the experimental value obtained would be 1.11 x 106 cpm. To convert this experimental reading into true dpm’s, it would be necessary to measure a standard sample of the isotope of interest, of known dpm’s. This information would permit the calibration of the efficiency of the instrument and the ready conversion of the cpm values of samples into dpm units.
When radiolabelled substrates are used in enzyme kinetic studies, the labelled substrate is usually mixed with “cold” (i.e., un-labelled) substrate to achieve a particular total substrate concentration without having to use high quantities of radioactivity. It is important, however, to quantify the proportion of radiolabelled molecules in the substrate sample.
Quantification is commonly expressed in terms of the specific radioactivity of the sample. (Note: “Specific activity” in this case refers to the radioactivity of the sample and should not be confused with the specific activity of an enzyme sample, which is defined later.) Specific radioactivity is given in units of radioactivity per mass or molarity of the sample.
Common units of specific radioactivity include dpm/ mmol and mCi/mg. With the specific radioactivity of a substrate sample defined, one can easily convert into velocity unit’s radioactivity measurements taken during an enzymatic reaction.
Good bookkeeping is essential in these assays. The amount of total substrate used will be dictated by the purpose of the experiment and it’s Km for the enzyme. The specific radioactivity, on the other hand, should be adjusted to ensure that the amount of radioactivity used is the minimum that will provide good signal-over-background readings.
Guidelines for sample preparation using different radioisotopes can be found in the reviews by Oldham (1968, 1992). The other point that should be kept in mind is that good post reaction separation of the labelled substrate and product molecules is critical to the use of radiolabels for following enzyme kinetics.
Radiolabelled substrates are commonly used in conjunction with chromatographic and electrophoretic separation methods. When the substrate and/or the product is a protein, as in some assays for kinases and proteases, bulk precipitation or capture on nitrocellulose membranes can be used to separate the macromolecule from the other solution components.
Errors in Radioactivity Measurements:
Aside from errors associated with bookkeeping, the most commonly encountered cause of inaccurate radioactivity measurements is self-absorption. When the separation method used in conjunction with the assay involves a solid separation medium, such as paper or thin-layer plate chromatography, gel electrophoresis, or capture on activated charcoal, the solid material in the sample may absorb some of the emitted radiation, preventing the signals from reaching the detection device.
This self-absorption is best corrected for by measuring all samples and standards at a constant density in terms of milligrams of material per milliliter. Segel (1976) suggests using an inert material such a gelatin to adjust the density of all samples for this purpose. Because scintillation counting measures light emission, the same interferences discussed for fluorescence measurements can occur.
In particular, if the sample is highly colored, quenching of the signal due to the equivalent of an inner filter effect may be observed When possible, this should be correct for by adjusting the optical density of the samples and standards with a similarly colored inert material (Segel, 1976).
Method # 5. Other Detection Methods:
Absorption, fluorescence, and radioactivity are by far the most common means of following enzyme kinetics, but a wide variety of other techniques have been utilized as well. Immunologic detection, for example, has been applied to follow proteolytic cleavage of a protein substrate by Western blotting, using antibodies raised against that protein substrate.
Recently, antibodies have been developed that react exclusively with the phosphorylated forms of peptides and proteins; these reagents have been widely used to follow the enzymatic activity of the kinases and phosphatases using Western and dot blotting as well as ELISA-type assays. Reviews of immunologic detection methods can be found in Copeland (1994) and in Harlow and Lane (1988).
Polarographic methods have also been used extensively to follow enzyme reactions. Many oxidases utilize molecular oxygen during their turnover, and the accompanying depletion of dissolved O2 from the solutions in which catalysis occurs can be monitored with an O2-specific electrode.
Very sensitive pH electrodes can be used to follow proton abstraction or release into solution during enzyme turnover. Enzymes that perform redox-chemistry as part of their catalytic cycle can also be monitored by electrochemical means. Reviews of these methods can be found in the text by Eisenthal and Danson (1992).
The variety of detection methods that have been applied to enzyme activity measurements is too broad to be covered comprehensively in any one volume. Our discussion should provide the reader with a good overview of the more common techniques employed in this field. The references given can provide more in-depth accounts.
Another very good source for new and interesting enzyme assay methods, the journal Analytical Biochemistry (Academic Press), has historically been a repository for papers dealing with the development and improvement of enzyme assays. Finally, the series Methods in Enzymology (Academic Press) comprises volumes dedicated to in-depth reviews of varying topics in enzymology.
This series details assay methods for many of the enzymes one is likely to work with and very frequently will indicate at least an assay for a related enzyme that can serve as a starting point for development of an individual assay method.