ADVERTISEMENTS:
Effect of Enzyme on Reaction Rate:
Two general mechanisms are involved in enzyme catalysis. First, the presence of the enzyme increases the likelihood that the potentially reacting molecular species will encounter each other with the required orientations in space.
This occurs because the enzyme has a high affinity for the reactants (more appropriately referred to as the substrates) and temporarily binds them in close proximity.
ADVERTISEMENTS:
The association of substrates with an enzyme is not arbitrary; the substrates are bound to the enzyme in such a manner that each substrate is oriented with respect to the other in precisely the manner required for the reaction to occur.
Second, the formation of temporary bonds between the enzyme and substrate forces a redistribution of electrons within the substrate molecules, and this redistribution imposes a strain on specific covalent bonds within the substrates—a strain that culminates in bond breakage. Biochemists refer to the introduction of bond strains in a substrate by association with an enzyme as “substrate activation.”
The net effect of this is to greatly increase the percentage of molecules in the population that at any instant are sufficiently reactive toward each other.
For reversible enzyme-catalyzed reactions such as:
In this equation, e is the “enzyme factor”—a factor that accounts for the increase in reaction rate through catalysis—and is proportional to the concentration of the enzyme in solution. That is, if the reaction proceeds at some rate at a given enzyme concentration, then the reaction would proceed twice as rapidly at twice the enzyme concentration (Fig. 8-3).
For the reverse reaction,
It can be shown using equations 8-13 and 8-14 that, the equilibrium constant for the reaction (i.e., k1/k2) is not altered by the presence of the enzyme. Thus, the enzyme greatly affects (i.e., increases) the rate at which equilibrium is achieved, but it does not alter the respective equilibrium concentrations of the various molecular species.
The marked effect of enzymes on reaction rates can be clearly appreciated by considering the turnover number of an enzyme. The turnover number is the number of moles of substrate converted to product per minute per mole of enzyme (when, the enzyme is fully saturated with substrate).
For example, the hydrolytic enzyme urease, which catalyzes the conversion of urea to ammonia, has a turnover number of about 106. In the absence of urease, the hydrolysis of urea is several orders of magnitude slower. Amylase, an enzyme involved in the breakdown of starch and other polysaccharides, can hydrolyze about 10 glycosidic bonds per minute, but no detectable hydrolysis occurs in the absence of amylase. The turnover” numbers of most enzymes fall between 102 and 106 (Table 8-1).
ADVERTISEMENTS:
Organization of Specific Enzymes:
Extensive studies during the past decade have revealed the precise atomic structure-of a number of important enzymes including lysozyme, ribonuclease, chymotrypsin, trypsin, carboxypeptidase, papain, elastase, and thrombin. A consideration of some of these reveals certain generalizations about the organization of enzyme molecules and provides insight into the mechanisms by which catalysis is achieved.
Lysozyme:
Lysozyme is an enzyme that is produced in both plant and animal tissues and was the first to have its complete three-dimensional structure revealed (by C. C. F. Blake, D. C. Phillips, and A. C. T. North, in 1965). The enzyme cleaves polysaccharide chains found in the cell walls of certain bacteria by hydrolyzing the glycosidic bonds between neighboring hexosyl residues.
ADVERTISEMENTS:
Egg white lysozyme has a molecular weight of about 14,600 and consists of a single polypeptide chain of 129 amino acids. The enzyme is oval in shape with a deep cleft across its midline that divides the molecule into two parts. The shape of lysozyme and the orientation of the substrate are depicted stereoscopically in Figure 8-16.
The polypeptide contains three short helical regions and a segment arranged in the form of a beta pleated sheet. All the polar residues are located on the enzyme’s surface and nearly all uncharged residues are buried internally. Hydrogen bonds between one portion of the polypeptide chain and another are pronounced and appear to be vital in sustaining the active tertiary structure of the molecule.
The cleft at the center of the molecule contains the active site and is studded with hydrophobic groups that probably form van der Waals bonds with the substrate. The bacterial polysaccharide that acts as substrate for the enzyme consists of chains of N- acetyl glucosamine (NAG) and N-acetylmuramic acid (NAM) in which the two sugars alternate. In the bacterial cell wall, these chains are cross-linked by short polypeptides.
ADVERTISEMENTS:
Six hexosyl units of the substrate polysaccharide are simultaneously bound at the active site of lysozyme. A change in tertiary structure, which takes the form of a narrowing and deepening of the cleft in the enzyme, accompanies substrate binding and forces a modification of the conformation of one of the six hexosyl groups (i.e., NAM) bound at the active site. This conformational change involves a distortion of the chair form normally assumed by this sugar and primes this region of the substrate for catalytic alteration. It is the glycosidic bond between this group and its neighbor that is broken during catalysis.
Of the 19 or more amino acids that compose the active site, only two have been identified as catalytic; these are asp (amino acid no. 52) and glu (amino acid no. 35). The remaining 17 residues align the substrate through hydrogen bonds and nonpolar interactions.
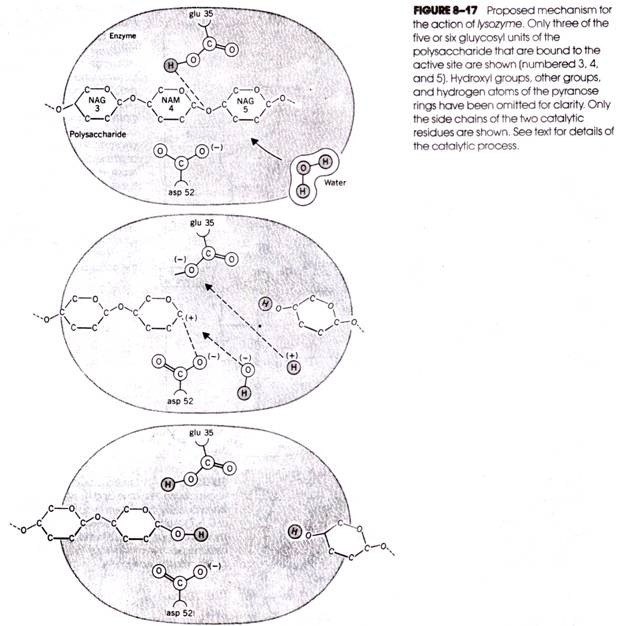
Asp 52 carries a dissociated carboxyl group (i.e., negative charge), but glu 35, being surrounded by the nonpolar side chains of other residues, is protonated. The sequence of events occurring during catalysis is depicted in Figure 8-17 in which only three of the six hexosyl units bound to the active site (i.e., numbers 3, 4, and 5) are shown.
The binding of the substrate to the enzyme and the conformational changes that occur in both as a result are followed by an attack by a hydrogen ion of the un-dissociated COOH group of glu 35 on the oxygen atom, forming the bridge between hexosyl units 4 and 5 (Fig. 8-17a).
ADVERTISEMENTS:
This, of course, is the specific region of the substrate placed under strain during binding. The glycosidic bond is broken, leaving carbon atom no. 1 of hexosyl unit 4 with a positive charge (i.e., a carbonium ion) (Fig. 8-176). The carbonium ion is stabilized by the formation of a temporary ionic bond with the dissociated side chain of asp 52 (Fig. 8-17b). The dissociation of a neighboring molecule of water provides a hydroxyl group for attack on the carbonium ion and a hydrogen for glu 35 (Fig. 8-17c). Once the reaction is completed, the two resulting polysaccharide fragments leave the active site.
Ribonuclease:
Ribonuclease is produced by the pancreas and secreted into the small intestine, where it catalyzes the hydrolytic digestion of polyribonucleotide chains. The enzyme consists of a single 124 amino acid polypeptide having a molecular weight of about 13,700. The reaction catalyzed by ribonuclease (shown in Fig. 8- 18) involves the hydrolytic cleavage of the ester linkage between the 3′-phosphate group of a pyrimidine nucleotide and the 5′ position of the adjacent nucleotide.
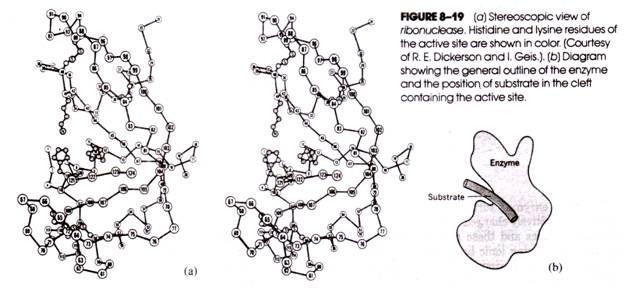
Like lysozyme, the active site of ribonuclease lies in a cleft in the enzyme’s surface. The cleft is lined by a number of positively charged side chains of lysine and arginine residues and these probably act as contact residues by forming ionic bonds with the negatively charged phosphate groups of the ribonucleic acid backbone (Fig. 8-18).
Projecting into the cleft from either side are two histidine residues that are believed to hydrolyze the RNA substrate by general acid-base catalysis in which the P—0 bond linking the ribose of one nucleotide with the ribose of a neighboring nucleotide is broken. Ribonuclease is depicted stereoscopically in Figure 8-19.
Chymotrypsin:
The digestive enzyme chymotrypsin is derived from the inactive precursor polypeptide chymotrypsinogen produced in the pancreas and containing 245 amino acids. The activation of chymotrypsinogen involves the preliminary cleavage of four of its peptide bonds, resulting in three separate polypeptide chains cross- linked by two disulfide bridges; two dipeptides released in the activation process do not become a part of the functional enzyme. Chymotrypsin digests alimentary protein by hydrolyzing peptide bonds on the carboxyl side of amino acids with large hydrophobic side chains (i.e., phenylalanine, tyrosine, and tryptophan residues).
Unlike lysozyme and ribonuclease, the active site of chymotrypsin resides in a shallow dish-shaped cavity on the surface of this roughly spherical enzyme. In the active site is a hydrophobic pocket that is believed to act as the receptor of the hydrophobic side chain of the substrate amino acid residue and thereby imparts specificity to this enzyme.
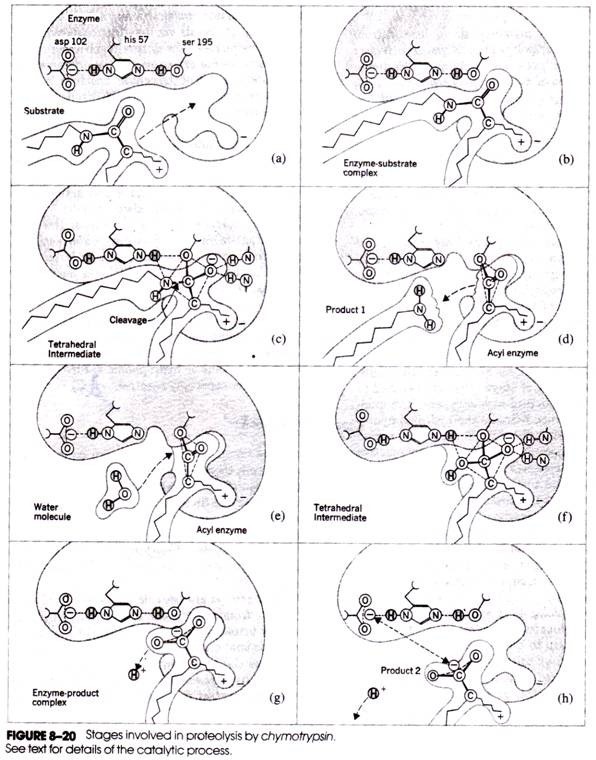
In addition to these and other contact residues, the active site contains three catalytic residues: asp 102, his 57, and ser 195. Figure 8-20 depicts the series of stages that are believed to be involved in the action of chymotrypsin. The imidazole group of histidine attracts the hydroxyl hydrogen atom of the serine, thereby rendering the serine oxygen strongly nucleophilic and particularly reactive toward amides and esters (Fig. 8-20a).
Binding of the substrate polypeptide to the enzyme (Fig. 8-20b) is followed by formation of a covalent bond between the serine oxygen and the a-carboxyl carbon atom of the substrate residue (Fig. 8-20c). This is followed by donation of the serine proton to the a-amino nitrogen atom of the neighboring substrate residue, the breakage of the peptide bond, and the release of the first product (Fig. 8-20d).
At this stage, a molecule of water enters the reaction (Fig. 8-20e), donating a hydrogen ion to serine and a hydroxyl group to the a-carboxyl carbon (Fig. 8-20f). Dissociation of this carboxyl group (Fig. 8- 20g) leaves the carboxyl group negatively charged. Repulsive forces between this group and the negatively charged aspartic acid residue of the active site facilitate separation of the enzyme and final product (Fig. 8-20 h).
The behavior of the catalytic serine residue is critical to the function of chymotrypsin. Other proteases, including trypsin, elastase, collagenase, thrombin, fibrinolysin, and some lysosomal proteases, share a similar overall tertiary structure and active site, and they cleave peptide bonds via the identical action of a catalytic serine residue.
The different substrate specificities of these serine proteases may be attributed to the nature and location of the contact residues in their active sites. In view of their remarkable similarity, the serine proteases are believed to have’ a common evolutionary origin.
The accumulated information on lysozyme, ribonuclease, and the serine proteases, as well as several other enzymes, permits certain generalizations to be made concerning enzyme structure and action. The active site of the enzyme nearly always resides in a depression in its surface, and the binding of the substrate to the active site is followed by conformational change in the enzyme, the substrate, or both.
The binding and/or the conformational changes that follow place a strain on certain bonds in the substrate and render them particularly susceptible to chemical attack. The catalysis itself may be facilitated by as few as one or two amino acid side chains of the enzyme, which, because of their spatial arrangement in the active site and the polar or hydrophobic nature of their surroundings, are especially reactive.
Cofactors:
Enzymes are composed of one or more polypeptide chains. However, there are a number of cases in which non-protein constituents called cofactors must be bound to the enzyme (in addition to the substrate) for the enzyme to be catalytically active. In these instances, the exclusively protein portion of the enzyme is called the apoenzyme. Three kinds of cofactors may be identified: prosthetic groups, coenzymes, and metal ions.
Prosthetic groups are organic compounds and are distinguished from other cofactors in that they are permanently bound to the apoenzyme. For example, in the peroxisomal enzymes peroxidase and catalase, which catalyze the breakdown of hydrogen peroxide to water and oxygen, heme is the prosthetic group and is a permanent part of the enzyme’s active site.
Coenzymes are also organic compounds, but their association with the apoenzyme is transient, usually occurring only during the course of catalysis. Furthermore, the same coenzyme molecule may serve as the cofactor in a number of different enzyme-catalyzed reactions. In general, coenzymes not only assist enzymes in the cleavage of the substrate but also serve as temporary acceptors for one of the products of the reaction. The essential chemical components of many coenzymes are vitamins.
For example, the coenzymes nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide -phosphate (NADP) contain the vitamin niacin; coenzyme A contains pantothenic acid; flavin adenine dinucleotide (FAD) contains riboflavin (i.e., vitamin B2); thiamine pyrophosphate contains thiamine (i.e., vitamin B,), and so on.
A number of enzymes require metal ions for their activity. The metal ions form coordination bonds with specific side chains at the active site and at the same time form one or more coordination bonds with the substrate. The latter assist in the polarization of the substrate bonds to be cleared by the enzyme.
For example, zinc is a cofactor for the proteolytic enzyme carboxypeptidase and forms coordination bonds with the side chains of two histidines and one glutamic acid residue at the active site. A fourth bond is formed between zinc and the a-carboxyl group of the substrate amino acids, and it is here that the cleavage of the peptide occurs. Table 8-4 contains a list of some of the cofactor-requiring enzymes.
The observation that catalytic activity is lost when an enzyme is stripped of its cofactor testifies to the crucial role played by these atoms or molecules.
This role is diverse:
1. In some cases, the cofactor completes the active site of the enzyme or modifies it in such a manner that substrate binding can ensue.
2. The cofactor acts as a donor of electrons or atoms to the substrate and following the reaction is returned to its former state.
3. Cofactors may also serve as temporary recipients of either one of the reaction products or simply an electron or proton, again being recycled to its former state some time after the main reaction is completed.
4. Finally, the cofactor, together with the side chains of residues at the active site, may serve to polarize the substrate and prime it for catalytic alteration.
The Regulation of Enzyme Activity:
Literally thousands of different enzyme-catalyzed reactions characterize the metabolism of a cell, and these are organized into a number of interconnected (branching and converging) pathways. It is clear that the orderly functioning of a cell or organism demands that controls be placed on these reactions so that specific metabolic pathways (and, therefore, specific cell functions) are active or operative at certain times and inactive at others. One way in which such regulation can be achieved is by altering the activity of specific enzymes under specific circumstances.
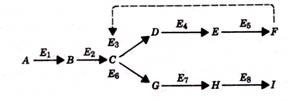
Consider as an example the following situation. Suppose that in the course of five enzyme-catalyzed reactions, substrate A is converted to end product F, that is, where E1, E2, E3, and so on are the enzymes involved in the pathway. If the end product F is able to bind to enzyme E1 and in so doing render the enzyme inactive (i.e., unable to catalyze the conversion of A to B), then the synthesis of end product F can be regulated, for under conditions of low F concentration, the metabolic pathway leading to F would be functioning, whereas at high concentrations of F, E1 inhibition by the end product would put a halt to the pathway leading to F. The phenomenon in which the end product of a metabolic pathway can regulate its own production by inhibition of this sort is called feedback inhibition.
Many metabolic pathways are characterized by a number of branch points. In the example below, substrate A may be converted either to end product F or end product I. In such a pathway, the enzyme affected by feedback inhibition would occur at the branch point, that is, enzyme E3 would be inhibited by end product F. Consequently, although the conversion of A to F would be inhibited, the formation of I would continue. The continued synthesis of I would not be possible if feedback inhibition occurred before the branch point.
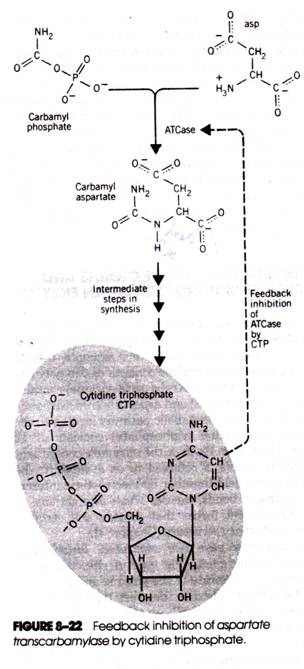
That the phenomenon of feedback inhibition actually occurs in cells was definitively established in the 1950s. One of the earliest discovered examples of feedback inhibition and a classic case is the effect of cytidine triphosphate (CTP, one of the final products formed during pyrimidine synthesis) on the enzyme aspartate transcarbamylase (Fig. 8-22).
The biosynthesis of CTP begins with the interaction of aspartic acid and carbamyl aspartate. The reaction is catalyzed by aspartate transcarbamylase (ATCase) and is followed by four more reactions, culminating in the formation of CTP. When the free CTP concentration is low (i.e., when CTP is being consumed in the cell’s metabolism), the formation of carbamyl asparate proceeds uninhibited. However, when the CTP level rises, carbamyl asparate synthesis is inhibited.
It is now clear that feedback inhibition is only one of several ways in which enzyme activity can be regulated. Not only end products but also other metabolites may be bound to an enzyme and in so doing alter the enzyme’s activity. The binding can take place at the active site or at other sites on the enzyme’s surface.
Indeed, in instances where the enzyme molecule has more than one active site, the binding of substrate at one site can influence subsequent substrate binding at another.

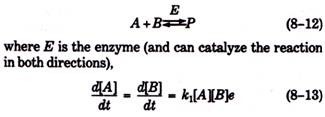


![clip_image002[6] clip_image002[6]](https://www.biologydiscussion.com/wp-content/uploads/2014/09/clip_image0026_thumb.jpg)