ADVERTISEMENTS:
The global Sulphur cycle is a good example to illustrate linkage between the air, water, and soil.
Like the nitrogen cycle, it also illustrates the key role played by microorganisms.
There are at least four major inputs of sulphur into the atmosphere from land— volcanic activity, soil dust, industrial activity and activity of sulphur bacteria which releases H2S into the atmosphere. Sulphur also enters the atmosphere from the oceans in three ways— deep sea hydrothermal vents, biogenic gas (dimethy1 sulfide) and sea water whipped by winds forms aerosols containing SO4.
ADVERTISEMENTS:
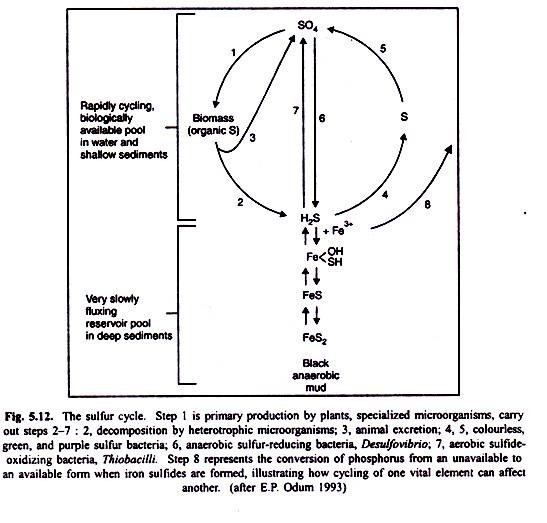
Plants and animals require a continuous supply of sulphur and its compounds in order to synthesize some amino acids such as cysteine and methionine, and proteins (Fig. 5.12). Only a few organisms gain their sulphur requirements in such organic forms as amino acids and cystein. Inorganic sulphate (SO4) is the major source of biologically significant sulphur.
Most of the biologically incorporated sulphur is mineralized by bacteria and fungi in ordinary decomposition by species of Aspergillus and Neurospora, among others. Under anaerobic condition, however, some may be reduced directly to sulphides, including hydrogen sulphide, by bacteria such as Escherichia and Proteus as shown in the following reaction: SO4——>H2S (anaerobic sulphate reduction).
Some organic sulphur enters the atmosphere as sulphur dioxide (SO2) through incomplete combustion of fossil fuels, especially coal. This is one of the major sources of air pollution today. According to Schindler (1988), anthropogenic inputs of sulphur in industrial areas may contribute 90% of the sulphur input into the air.
ADVERTISEMENTS:
Most of the input is in the form of SO2 which, in the presence of water, may be converted to HSO3 or SO4 by the following reactions:
SO2——-> SO3 —–> H2SO4⥢ H+ + SO24–
SO2——–>H+ + HSO3
The deposition of SO2-4 HSO3 and H+ ions lowers the pH of precipitation, causing acid rain problem. Inorganic sulphur as sulphate may precipitate out, but since it is relatively soluble, it serves as a source of elemental sulphur in many ecosystems. Sulphate is also reduced under anaerobic conditions to elemental sulphur or to sulphides including hydrogen sulphide, by such heterotrophic bacteria as species of Desulfovibrio and Desulfomonas (SO2-4 ——> S2). Colourless sulphur bacteria such as species of Beggiatoa (chemosynthetic bacteria) often abundant in sulphur springs oxidize hydrogen sulphide to elemental sulphur, and species of Thiobacillus oxidize it to sulphate.
Other species of Thiobacillus oxidize sulphide to sulphur, and still others oxidise sulphur to sulphate as shown in the following reactions:
H2S —->S——->SO4
H2S—->SO4
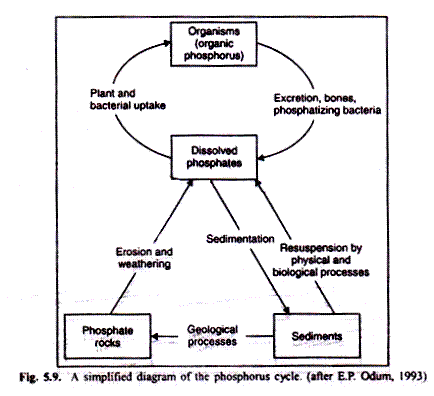
The sedimentary aspect of the sulphur cycle involves the precipitation of sulphur in the presence of iron under anaerobic conditions. Ferrous sulphide is insoluble in neutral or alkaline water, and consequently sulphur has the potential for being bound up under these conditions to the limits of the amount of iron present.
Because of the thermodynamics of this ferrous sulphide (FeS) system, other nutrients important to biological systems, can also get frapped for varying periods of time. Among these are copper, cadmium, zinc and cobalt. When iron sulphides are formed in the sediments, phosphorus is converted from insoluble to soluble form (see “phosphorus release” arrow in Fig. 5.9) and thus becomes available to organisms. This is an excellent example of how one cycle regulates another.