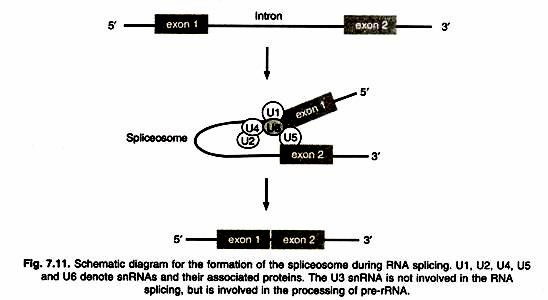
ADVERTISEMENTS:
Some of the important types of Biogeochemical Cycles: Hydrologic Cycle, Gaseous Cycles and Sedimentary Cycles!
Nearly 30 to 40 elements are required for proper growth and development of living organisms.
Most important of these are C, H, O, P, K, N, S, Ca, Fe, Mg, B, Zn, CI, Mo, Co, I and F. These materials flow from abiotic to biotic components and back to the non-living component again in a more or less cyclic manner.
ADVERTISEMENTS:
This is known as the biogeochemical cycle or inorganic-organic cycle. The flow of these elements through the ecosystem must be cyclic, with matter being consistently reused. Because the flow involves not only the living organisms but also a series of chemical reactions in the abiotic environments, these cycles are called biogeochemical cycles.
There are three types of biogeochemical cycles:
(1) Hydrologic cycle or water cycle,
ADVERTISEMENTS:
(2) Gaseous cycles, and
(3) Sedimentary cycles.
1. Hydrologic or Water Cycle:
Interchange of water between atmosphere, land and sea and between living organisms and their environment is accomplished through water cycle. Water cycle or hydrologic cycle involves evaporation, transpiration, cloud formation and precipitation. Water of atmosphere reaches the earth surface through precipitation and from the earth surface it reaches the atmosphere through evaporation and transpiration. The amount of water available for evaporation is determined by the amount supplied by precipitation and condensation. Between rainfall input and evaporation output there lies a precarious water balance (Fig. 3.16).
2. Gaseous Cycles:
Oxygen cycle:
Oxygen is found in free state in atmosphere and in dissolved state in water It is liberated as by-product of photosynthesis and is utilized in respiration by the plants and animals. When the living organisms respire, CO2 is liberated which is utilized by green plants as an essential raw material for carbohydrate synthesis. In this way, a simple yet vital O cycle IS maintained in the ecosystem (Fig. 3.17).
Carbon cycle:
Carbon is the basic constituent of all organic compounds. Since energy transfer occurs in the consumption and storage of carbohydrates and fats, carbon moves to the ecosystem with flow of energy The source of nearly all carbon found in the living organisms is CO2 which is found in free state in atmosphere and in dissolved state in the water on the earth. Green plants (producers) use CO2 through photosynthesis in the presence of sunlight and carbohydrate is formed. Later on, complex fats and polysaccharides are formed in plants which are utilized by animals.
Flesh eating animals (carnivores) feed on herbivores and the carbon compounds are again digested and converted into the other forms. Carbon is released to the atmosphere directly as CO2 in respiration of both plants and animals.
Bacteria and fungi attack the dead remains of plants and animals. They degrade the complex organic compounds into simple substances which are then available for other cycles. Part of the organic carbon is incorporated into the earth’s crust as coal, gas, petroleum, limestone and coral reef Carbon from such deposits may be liberated after a long period of time (Fig. 3.18).
Nitrogen cycle:
Of all the elements which plants absorb from the soil, nitrogen is the most important for plant growth. This is required in greatest quantity. Nitrogen is required for the synthesis of amino acid, proteins, enzymes, chlorophylls, nucleic acids, etc.
ADVERTISEMENTS:
Green plants obtain nitrogen from the soil solution in the form of ammonium, nitrate and nitrite ions and the main source of all these nitrogen compounds is the atmospheric nitrogen. The atmospheric nitrogen is not directly available to the organisms with the exception of some prokaryotes like blue green algae and nitrogen fixing bacteria.
Nitrogen cycle consists of the following steps:
(1) Nitrogen fixation,
ADVERTISEMENTS:
(2) Nitrogen assimilation,
(3) Ammonification,
(4) Nitrification,
(5) Denitrification, and
ADVERTISEMENTS:
(6) Sedimentation.
1. Nitrogen fixation:
Conversion of free nitrogen of atmosphere into the biologically acceptable form or nitrogenous compounds is referred to as nitrogen fixation.
This process is of two types:
ADVERTISEMENTS:
(a) Physicochemical or non-biological nitrogen fixation
(b) Biological nitrogen fixation.
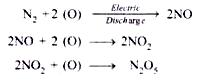
In physicochemical process of nitrogen fixation, atmospheric nitrogen combines with oxygen (as ozone) during lightning or electrical discharges in the clouds and produces different nitrogen oxides:
The nitrogen oxides get dissolved in rain water and on reaching earth surface they react with mineral compounds to form nitrates and other nitrogenous compounds:
Biological nitrogen fixation is carried out by certain prokaryotes. Some blue-green algae fix significant amounts of nitrogen in the oceans, lakes and soils. Symbiotic bacteria (Rhizobium) inhabiting the root nodules of legumes (Fig. 3.19) and also the species of alder, buck brush and a number of other non-leguminous genera and symbiotic blue-green algae (species of Nostoc Anabaena, etc.) found in free state or in the thalli of Anthoceros, Salvenia, Azolla, coralloid roots of Cycas fix atmospheric nitrogen. The relation is mutualistic because the microbes use energy from the plants to fix nitrogen that is made available to the host plants and other plants of the community.
Certain free living nitrogen fixing bacteria, such as Azotobacter, Clostridium Beijermckia. Derxia. Rhodospirillium also fix free nitrogen of atmosphere in the soil. Frankia an actmomycetous fungus found in the roots of Alnus, Percia, Casuarina, etc. also fixes nitrogen. Nitrogen fixing organisms combine the gaseous nitrogen of atmosphere with hydrogen obtained from respiratory pathway to form ammonia which then reacts with organic acids to form aminoacids. Biological nitrogen fixation is the major source of fixed nitrogen upto 140—700 mg/m2/year as against 35 mg/m2/year by electrical discharge and photochemical fixation.
2. Nitrogen assimilation:
Inorganic nitrogen in the form of nitrates, nitrites and ammonia is absorbed by the green plants and converted into nitrogenous organic compounds. Nitrates are first converted into ammonia which combines with organic acids to form amino acids. Amino acids are used in the synthesis of proteins, enzymes, chlorophylls, nucleic acids, etc. Animals derive their nitrogen requirement from the plant proteins. Plant proteins are not directly utilized by the animals. They are first broken down into amino acids during digestion and then the amino acids are absorbed and manipulated into animal proteins, nucleic acids, etc.
3. Ammonification:
The dead organic remains of plants and animals and excreta of animals are acted upon by a number of microorganisms especially actinomycetes and bacilli (Bacillus ramosus, B. vulgaris, B. mesenterilus). These organisms utilize organic compounds in their metabolism and release ammonia.
ADVERTISEMENTS:
4. Nitrification:
Certain bacteria, such as Nitrosomonas, Nitrococcus, Nitrosogloea and Nitrospira in oceans and soils convert ammonia into nitrites and then nitrites into nitrates. These bacteria primarily use the energy of dead organic matter in their metabolism.
2NH4++ 202 →NO2– + 2H2O + energy
Conversion of nitrites to nitrates is brought about by several microbes like Penicillium species, Nitrobacter, Nitrocystis etc. Nitrocystis oceanus is the common marine autotroph which performs nitrification for obtaining energy.
2NO-2 + O2 → 2NO3– + energy
Some nitrates are also made available through weathering of nitrate containing rocks.
5. Denitrification:
Ammonia and nitrates are converted into free nitrogen by certain microbes. This process is referred to as de-nitrification. Thiobacillus denitrificans, Micrococcus de-nitrificans, Pseudomonas aeruginosa are the common examples of denitrifying bacteria.
2NO-3→ 2NO-2→2NO→ N2O→N2
6. Sedimentation:
Nitrates of the soil are washed down to the sea or leached deep into the earth along with percolating water. Nitrates thus lost from the soil surface are locked up in the rocks. This is sedimentation of nitrogen. Nitrogen of rock is released only when the rocks are exposed and weathered.
Thus a large part of nitrogen is fixed up and stored in plants, animals, and microbes. Nitrogen leaves the living system in the same amount it is taken in from the atmosphere and the input and outflow of nitrogen are balanced in the ecosystem. The overall nitrogen cycle in nature v presented in Fig. 3.20.
3. Sedimentary Cycles:
Mineral elements required by living organisms are obtained initially from inorganic sources. Available forms occur as salts dissolved in soil water.
Mineral cycles essentially consist of two phases:
(i) The salt solution phase, and
(ii) Rock phase.
Mineral salts come directly from earth crust by weathering. Soluble salts then enter the water cycle. By movement of water minerals move from the soil to streams, lakes and ultimately to sea where they remain permanently. Other salts return to the earth’s crust through sedimentation. They become incorporated into sediments or rock beds and after weathering of rocks they again enter the cycle.
Plants and some animals take minerals in the form of mineral solution from their habitats. After the death of living organisms the nutrients return to the soil and water through the action of decomposers (bacteria and fungi) and transformers. Green plants at one end and decomposers at the other play very important role in circulation of nutrients.
Phosphorus cycle:
Plants and animals obtain phosphorus from the environment. Phosphorus is a component of nucleic acids, ADP, ATP, NADP, phospholipids etc. It occurs in the soil as rock phosphate, apatite or calcium phosphate, fluorapatite [Ca10Fe2 (PO4)6], iron phosphate or aluminium phosphate. Soils derived from the rock beds rich in phosphates are rich in phosphorus.
Phosphorus occurs in the soil in five forms P1 (stable organic), P2 (labile organic), P3 (labile inorganic), P4 (soluble) and P5 (mineral form) and of these forms, P3 and P4 are in equilibrium and entry of phosphorus in the green plants is considered to occur via labile inorganic pool.
The dissolved phosphorus is absorbed by plants and converted into organic form. From plants it travels to various trophic levels in the form of organic phosphates. When the plants and animals die the decomposers attack them and liberate phosphorus to the environment. Thus, this process proceeds in cyclic way. A general picture of the phosphorus cycling is presented in Fig. 3.21.
Phosphorus along with many other mineral elements reaches the oceans and settles down as sediment. A good proportion of phosphorus leaches down to deep layers of soil. In this way, major proportion of phosphate becomes lost to this cycle by physical processes, such as sedimentation and leaching. Biological processes such as formation of teeth and bones also keep phosphorus locked up for some time.
Sulphur Cycle:
Sulphur cycle links soil, water and air. Sulphur occurs in the soil and rocks as sulphides (FeS, ZnS, etc.) and crystalline sulphates. In the atmosphere sulphur occurs in the form of SO2 and H2S. SO2 gas is formed during combustion of fossil fuels or as a result of decomposition. H2S or hydrogen sulphide gas is released to the atmosphere from water logged soils, continental shelf, lakes and springs. The organic and inorganic sulphur and SO2 are formed through oxidation of H2S in the atmosphere.
A small amount of sulphur occurs in dissolved state in rain water and through rains it reaches earth surface. Except a few organisms which need organic form of sulphur as amino acids and cysteine, most of the organisms take sulphur as inorganic sulphates. Most of the biologically incorporated sulphur is produced in the soil from aerobic breakdown of proteins by bacteria and fungi. Under an aerobic condition, however, sulphur may be reduced directly to sulphides, including H2S.
Green and purple photosynthetic bacteria use hydrogen of H2S as the oxygen acceptor in reducing carbon dioxide. Green bacteria are able to oxidise sulphide to elemental sulphur whereas the purple bacteria can carry oxidation to sulphate stage. In the ecosystems, sulphur is transferred from autotrophs to animals, then to decomposers and finally it returns to environment through the decay of dead organic remains (Fig. 3.22).
Sedimentary aspect of sulphur cycling involves precipitation of sulphur in presence of iron under anaerobic conditions. Sulphides of iron, copper, zinc, cadmium, cobalt are insoluble in neutral and alkaline water and consequently sulphur is bound to limit the amount of these elements. Thus, sulphur cycle affords an excellent example of interaction and complex biochemical regulation between the different mineral cycles.
The study of biogeochemical cycles in the ecosystem makes it clear that the abiotic components of ecosystem are transformed into biotic structures through metabolic processes and locked up in the biomass for some time depending upon the turnover rate. In lower plants with soft tissues the turnover rate is quicker than in higher plants and animals. The materials held up in the biomass are released to the environment by decomposing activities.
The nutrient cycle is not a close circuit within an ecosystem. The nutrients are continuously being imported as well as carried out of the ecosystem. Appreciable quantities of plant nutrients are brought to ecosystem by rain and snow. Small quantity of nutrients is carried to the forest by rains. The gain nutrients to the ecosystem from precipitation, extraneous material and mineral weathering is offset by losses.
Water draining away from forest carries with it more mineral matter than supplied through precipitation. Considerable quantities of nutrients in the forest are locked up in the trees and the humus layer. When trees and vegetation are removed sufficient amounts of nutrient are removed. Intensive forestry and agriculture on some soils may reduce the nutrient reserves to such an extent that soils become unfertile. Ecosystem can remain productive only It the nutrients withdrawn are balanced by an inflow or replacement.