ADVERTISEMENTS:
The following points highlight the twelve various types of proteins that regulates the immune system. Some of the types are: 1. Immunoglobulins 2. Immunoglobulin Classes 3. Antibody Variants 4. Mechanism of Antibody Diversity 5. Cytokines 6. Complement 7. T-Cell Receptor 8. Major Histocompatibility Complex (MHC) or Human Leukocyte Antigen (HLA) 9. Adjuvant 10. Autoimmunity and Others.
Type # 1. Immunoglobulins:
The immunoglobulin’s are a group of glycoproteins present in the serum and tissue fluids of all mammals.
They are classified into two categories:
ADVERTISEMENTS:
i. Some are carried on cell surfaces where they act as receptors.
ii. Some are free in the blood or lymph. These are called antibodies.
The immunoglobulin’s which act as receptors, are mainly found on the membrane of T lymphocytes where there is no production of soluble molecules, analogous to the circulating antibodies. T cell recognition of antigen is the basis of a range of immunological phenomena including T cell helper and suppressor activity, cytotoxicity and possibly natural killer activity.
Antibodies (Ab) are also produced in large quantities by B lymphocytes. In fact they are the soluble form of B cell’s antigen receptors. In general, contact between these B cells and a foreign antigen is required for intracellular induction of antibody formation which is specific for that particular antigen.
ADVERTISEMENTS:
The antibody thus produced binds specifically to the stimulating antigen. Binding of the antibody to the antigen does not cause the destruction of the antigen. Rather, the antibody serves to mark and identify the target and to activate immune responses that can destroy the target.
Thus bacteria covered by antibodies are better target for phagocytosis by neutrophils and macrophages. Actually, each antibody molecule is bi-functional. One region of the molecule is concerned with binding to antigen while a different region mediates so- called effector functions. This function includes binding of antibody to some phagocytic cells of the immune system as well as the components of complement system.
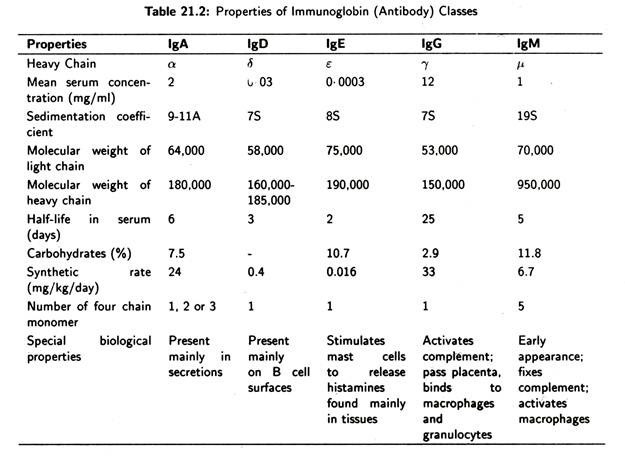
Five distinct classes of immunoglobulin molecules—IgG, IgA, IgM, IgD and IgE-have been identified in the serum and tissue fluid of the higher animals. These differ in size, charge, amino acid composition and carbohydrate content.
In addition to the difference between classes, the immunoglobulin’s within each class are also very heterogeneous. Serum glycoproteins on subject to electrophoresis get separated according to their charge into various groups like albumins, α1 globulin, α2 globulin, β globulin and y globulin.
Gamma globulin is composed of a heterogeneous group of immunoglobulin’s—IgG, IgA and IgS. The mobility of IgM and IgD is restricted in the β and fast y region. The IgE class has a similar mobility to IgD but cannot be represented quantitatively because of its low level in serum.
Each immunoglobulin molecule is made of two kinds of chains, light (L) chain (MW = 23,000) and heavy (H) chains (MW = 53,000 or more). There are two types of L chains called kappa (k) and lambda (λ) chains, each antibody molecule can have either k chains or A chains, but not both types.
H chains are of five types. IgG have 7 chains. IgM have µ chains and the others have δ, ɛ or α chains (Table 21.2). Out of five distinct classes of immunoglobulin’s two major classes (IgG and IgA) have subclasses. There are four subclasses of human IgG—IgG1, IgG2, IgG3 and IgG4—which have y1, y2, y3 and y4 heavy chains, respectively.
There are also two subclasses of IgA such as IgA1 and IgA2 which have α1 and α 2 heavy chains, respectively. IgM, IgD and IgE have no subclasses. Asparagine linked carbohydrate chains are also attached to all H chains.
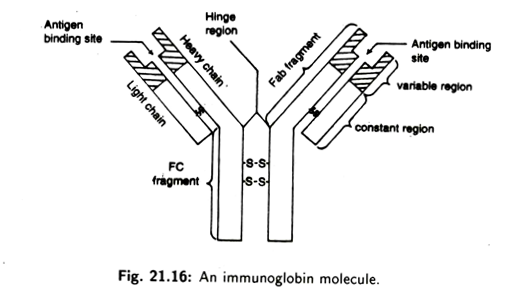
Every individual antibody molecule has two identical L chains and two identical H chains. The four chains are linked together by disulphide bonds (Fig. 21.13). Each light chain usually consists of about 220 amino acids and each heavy chain usually consists of 440 amino acids. Within each chain, units made of about 110 amino acids fold up to form compact loop called domains (Fig. 21.14).
Two ends of a loop (domain) are held together by a single internal disulphide bond. An L chain have two domains; H chains has four or five domains.
Both light and heavy chains are composed of two distinct regions (Fig. 21.15):
i. variable regions (VL and VH);
ii. constant regions (Cl and Ch).
The four chains of immunoglobulin’s axe arranged in the form of a flexible ‘Y’-shape of which the two upper arms are made of an inner H chain and an outer L chain. The stalk of Y is made of only two H chains. The stalk of Y is also called crystallisable fragment (Fc) and contains the site at which the antibody molecules combine to a cell.
ADVERTISEMENTS:
The arms of the Y- shaped antibody consist of two antigen-binding fragments (Fab) that bind with compatible epitopes of an antigen.
The Fc is composed of only constant region of heavy chain whereas the Fab has the variable region of both light and heavy chains. At the junction of the two arms with stalk, in most H chains a hinge region is found. The hinge is flexible and allows the antigen binding regions to move freely relative to the rest of the molecule.
Type # 2. Immunoglobulin Classes:
There are five distinct classes of immunoglobulin’s in most higher mammals. These are IgG, IgM, IgA, IgD and IgE. Each immunoglobulin class has a characteristic type of heavy chain. Thus IgG possesses y chains, IgM µ chains, IgA α chains, IgD δ chains and IgE e chains. Variation in heavy chain structure within a class gives rise to immunoglobulin subclasses.
The properties of the five immunoglobulin classes are summarised in Table 21.2.:
(a) IgG:
This is the major immunoglobulin in human serum constituting 70-75% of the total immunoglobulin pool. IgG is present in blood plasma and tissue fluids. It acts against bacteria and viruses by opsonizing them and neutralizing toxins. It is also one of the two immunoglobulin classes that can activate the complement system by the classical pathway.
IgG is the only immunoglobulin molecule capable to cross the placenta and provide naturally acquired passive immunity to the new born. The four subclasses of IgG—IgG1, IgG2, IgG3 and IgG4 (Fig. 21.16)—occur in , the approximate proportion of 66%, 23%, 7% and 4%, respectively.
(b) IgM:
This accounts for about 10% of immunoglobulin pool. It is a pentamer of five inonomeric units, each composed of two heavy and two light chains. The monomers are arranged in a wheel like fashion with the Fc and towards the centre held together by a special J (joining) chain (Fig. 21.17).
ADVERTISEMENTS:
IgM is the first immunoglobulin secreted into serum during primary antibody response. Since IgM is large, it does not leave the blood serum or cross the placenta. IgM agglutinates bacteria, enhance the ingestion of pathogen by phagocytic cells.
(c) IgA:
This accounts for about 15% of the immunoglobulin pool. In man, more than 80% of IgA is present in the serum as a monomer of two heavy and two light chains (Fig. 21.18), but in most mammals the IgA in serum is mainly polymeric dimer held together by J chain (Fig. 21.19).
IgA has special features that are associated with secretory mucosal surfaces, it acquires a protein termed as secretory component. Secretory IgA (slgA) or the modified molecule is now called primary immunoglobulin of the secretory immune system.
This system is found in the gastrointestinal tract, tracheobronchial tract and genitourinary system. Secretory IgA is also found in the saliva, tears and breast milk. slgA helps to protect newborns. In the intestine it attach o the ingested viruses, bacteria and pathogen adherence to mucosal surfaces and any invasion of host tissues the phenomenon is termed immune exclusion.
(d) IgD:
This is an immunoglobulin found in trace amounts in the blood serum. It is a monomer in its structure and shows similarity to IgG. They are capable of crossing the placenta and are abundant on the surfaces of B cells and they bind to antigens, (Fig. 21.20) IgD helps or activates B cells following antigen binding.
(e) IgE:
IgE (Fig. 21.21) represents only 0.0005% of the immunoglobulin pool. It is important because it can bind to cells containing potent pharmacologic mediators. When antigen binds to IgE, histamine is released. Histamine is responsible for many allergy symptoms.
IgE is also responsible for immunity to invading parasitic worms, helminth, parasites etc. It stimulates certain phenomena like gut hypermobility (increased rate of movement of intestinal content) that aid in elimination of helminthic parasites.
Type # 3. Antibody Variants:
Each subclass of IgG and IgA have differences in the amino acid composition of the heavy chain (y).
The variations may be classified as:
(a) Isotypes:
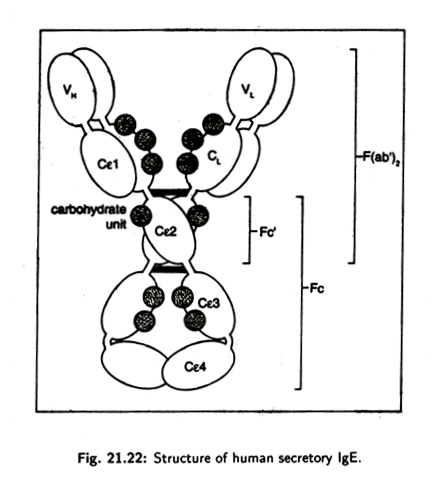
It refers to the variations in the heavy chain associated with the different classes and subclasses that are normally present in all individuals. The genes for isotypic variants are present in all healthy members of a species. For examples, the genes for y1, y2, y3, µ, α1,α2δ,ɛ, k and λ chains are present in the human genome and these axe, therefore, isotypes (Fig. 21.22).
(b) Allotype:
This type of variation is produced due to genetic variation between individuals within a species involving different alleles at a given locus. Allotypic variation is not found in all individuals. It occurs mostly as variants of heavy chain constant region (Fig. 21.22).
(c) Idiotype:
It referees to the variation in the highly variable region known as hyper-variable regions of the Fab region. These determine the binding specificity of the antigen binding site.
Idiotypes are of two categories:
i. Private Idiotype: These idiotypes axe usually specific for individual B cell clones.
ii. Recurrent Idiotype: Private idiotypes are sometimes shared between different B cell clones. This idiotype is also known as public idiotype or cross-reacting idiotype.
The specific genetic basis of idiotype variability is only partly known.
Type # 4. Mechanism of Antibody Diversity:
The most remarkable property of antibodies is their diversity. Current estimates indicate that a person synthesizes more than 106 different kind of immunoglobulin’s.
This diversity is largely the result of variations in the sequence of amino acids in the variable domains of both light and heavy chains and this is generated by the following mechanisms:
i. Genome rearrangements during B-lymphocytes differentiation.
ii. Alternate pathways of transcript splicing.
iii. Variable joining sites and somatic mutations.
Genome rearrangement generates antibody diversity. The genetic information coding for antibody chains is stored in bits and pieces in the germ cell’s DNA. These bits and pieces undergo extensive rearrangement by different permutation and combination only for variable regions of antibody. This occurs during the differentiation of B-lymphocytes from germ line cell.
Hence each B-lymphocyte receives non-identical genetic sequence for coding the variable region of both light and heavy chains of antibody molecule. As a result each B- lymphocyte produces only a single type of antibody and all the antibodies produced by a given B-lymphocyte have the same antigen- binding specificity.
(a) Diversity of Kappa Light Chains:
Synthesis of kappa light chain is regulated by three different gene segments such as VK, JK and CK. The arrangement of the kappa chain gene segments in germ line cells of human is shown in Fig. 21.24. In human all the kappa chain gene segments are located on chromosome 2.
In germ line cells there are about 300 VK gene segments, each with a nearby hK gene segment (codes for an N-terminal hydrophobic leader sequence, 17-30 amino acid long) [Fig. 21.24(a)]. On the other hand, there is only one CK gene segment.
The five JK segments are separated from the VK segments by long non- coding sequence and from the CK segment by another set long non-coding sequence. Thus during the development of a B-lymphocytes, the particular kappa light chain gene that will be functional in that cell is put together from the LK – VK segment, one JK segment and the single CK segment by a process of somatic recombination.
This process joins any one of the 300Lk – VK segments with any one of the five JK segments, while one CK gene segment is common for all. Thus this process yields the diversity of kappa light chains. In mature kappa light chain, the leader sequence is cleaved-off when it passes through the plasma membrane.
(b) Diversity of lambda light chain:
About 40% of the human antibody have lambda light chains. Lambda chain also undergoes variation due the genome rearrangement during B-lymphocyte development. In lambda chain, Lλ gene segment always comes with Vλ and joins to Jλ – Cλ gene segments. Mice have only four Jλ– Cλ gene segment where humans have six.
(c) Diversity of heavy chain:
The genetic organisation of heavy chain of antibody is more or less analogous to kappa light chain. It is composed of LH – VH JH and CH gene segments. But there is one additional gene segment called D which codes for 2-13 amino acids of the variable region.
In mouse germ line cells, as shown in Fig. 21.25, there are about 300 LH – VH segments 10-50 D gene, 4JH gene segments and 8Ch gene segments arranged on DNA.
During the development of B-lymphocyte one LH – VH gene segment joins with one D gene and one Jh gene segment to form one continuous DNA sequence that codes for entire heavy chain variable region.
The use of alternate sites of gene segment joining during the genomic rearrangement events and somatic hyper-mutation within the variable region gene segments contribute to the production of additional antibody diversity.
There are eight CH gene segments in mouse and 9 or 10 gene segments in humans. The CH gene segments such as CHµ, CHδ CHɛ CHα etc. code for the heavy chain constant region of IgM, IgD, IgE and IgA, respectively. The class of antibody is determined by its heavy chain constant region which, in turn, is determined by the C-gene segment that was expressed during its synthesis.
The phenomenon of class switching occurs when a B-lymphocyte stops synthesizing one class of antibody and begins synthesizing another class of antibody with the same antigen specificity. Class switching involves the expression of the same variable region gene segments but a different heavy chain constant region gene segment.
Hence it may produce IgM or IgG or IgE or IgA. Class switching most often occurs by further genomic rearrangements similar to those that resulted in the synthesis of the original antibody chain.
(d) Alternative pathway of transcript splicing:
Class switching during B-lymphocyte differentiation can also occur by alternate patterns of transcript splicing. Both IgM and IgD antibodies produced by some mature B-lymphocytes are transcribed as a single primary transcript from the DNA.
The primary transcript is encoded with Lh2 Vh2 D2 Jh4 gene sequence plus Chµ as well as Chδ gene segments. During processing the LH2 VH2 D2 Jh4 transcript sequence may be spliced to either the Ch„ gene segment or the Chs gene segment such that both types of heavy chains are synthesised in the same cell (Fig. 21.26).
Another type of splicing mechanism is found in developing B-lymphocyte during the sequential production of membrane-bound and secreted form of IgM molecule. These two forms of IgM differ only in the C terminal portion of their heavy chain.
The heavy chain of the membrane-bound form is 21 amino acids longer than that of the secreted form. The membrane-bound form has a 41 amino acid long hydrophobic sequence at the C terminal whereas the secreted form has 20-amino acid long hydrophilic sequence.
The exons (coding sequence) of the ChH gene segments of IgM antibody are interrupted by introns (non-coding sequences). The ChH gene segments contain four to six exons and three to five introns. In case of membrane-bound form of antibodies, the constant regions of heavy chain are produced by splicing all six exons together (Fig. 21.27) of which the last two exons code for the hydrophobic tails sequence.
During the synthesis of secreted form of IgM heavy chain, the constant regions of heavy chain are made of four ChH gene segments.
Due to lack of two ChH gene segments the product of secreted form of IgM will differ from membrane-bound form and makes the diversity.
(e) Variable Joining Sites and Somatic Mutation:
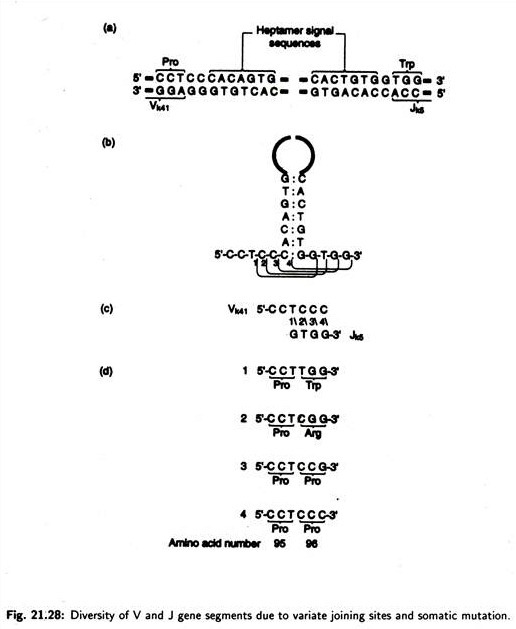
Besides genome rearrangement and transcript splicing, antibody diversity may be produced. This additional diversity could be explained by variation in the exact site of recombination during the V – J joining events. During the joining of gene segment, V and J recombination has been found to occur between four adjacent nucleotide positions at the junction sites.
The heptamer signal sequence plays an important role in bringing the V and J gene segments into juxtaposition. Four positions of joining and the products of the four alternate joining events have been shown in Fig. 21.28. Thus the use of alternate sites of recombination during the joining events involved in the assembly of mature antibody genes provides an additional mechanism for generating antibody diversity.
There is still another mechanism of antibody diversity. When the actual amino acid sequences of different λ1 chains of mouse were compared with predicted amino acid sequences based on the nucleotide pair sequences of λ gene segments of light chain, differences were observed in the variable regions away from the junction sites.
Similar observations have been made in studies of heavy chains at the same region. In all cases the changes have resulted due to substitutions of 1-2 nucleotide pairs of gene segments encoding the variable regions of antibody. These substitute may occur by somatic mutation. The process by which somatic mutation occur, is sometimes called somatic hyper mutation. The actual cause of hyper mutation is unknown.
Type # 5. Cytokines:
This is a generic term which is used to mean a large group of soluble molecules involved in signalling between cells during immune responses. Cytokines are proteins or peptides. Some cytokines are associated with sugar molecules to form glycopeptides.
There are different kinds of cytokines which fall into a number of categories such as:
i. Interferon’s (IFNS)
ii. Interleukins (ILs)
iii. Colony stimulating factors (CSFs)
iv. Tumour necrosis factors (TNFa and TNF»
i. Interferons:
The body’s most rapidly produced defense against viruses is a stable, non-toxic, potent and broadly active glycoprotein, secreted by body’s cells when they are stimulated by viruses, bacteria, foreign cells and several other macromolecules.
The secreted interferon then stimulate surrounding cells to produce other proteins which in turn may regulate virus multiplication, the immune response, cell growth and other cell functions. Interferon is to be abbreviated as ‘IFN’. At least three different types of interferon’s may be produced depending on the type of stimulus and type of cells stimulated. These are:
All the above interferon’s are coded by different structural genes as determined by their distinct amino acid sequence.
The amino acid sequences of IFNa CLASS have been determined completely and is found that it contains about 166 amino acids, starting with Cys-Asp- Leu and ending with Arg-Lys-Glu. IFN can be subdivided into five subclasses—IFN α 1, IFN α2, IFN α 3, and IFN α 4 and IFN α 5‘. Sequencing of other two interferon’s has not yet been done completely.
A list of inducers which can induce the cells for the production of interferon:
(a) Microorganisms:
(a) Virus: Major DNA virus group: Papo, Herpes, Pox, Adeno. Major RNA vims group: Picorna, Myxo, Arbo, Reo.
(b) Chlamydia;
(c) Rickettsia.
(d) Bacteria: Brucella, E. coli, Salmonella typhimurium, Serratia, Bordetella, Hemophillus, Listeria, Franscisella, and many others.
(b) Microbial Extracts:
(a) Nucleic acid extracts, dS. RNA from the viruses;
(b) Rickettsial extracts;
(c) Bacterial extracts, endotoxin, exotoxin, toxoids;
(d) Fungal extracts; and
(e) Plant extract like phytohemagglutinin from kidney bean, concanavalin etc.
(c) Synthetic Polymers:
(a) Polycarboxylates;
(b) Polysulfates, poly-vinyl-sulfates;
(c) Polyphosphates;
(d) Polythiophosphates and
(e) Poly (U) or Poly (C).
(d) Mechanism of Interferon Induction:
The mechanism involves de novo synthesis by de-repression of host DNA to form a messenger RNA for synthesis of the corresponding interferon molecule.
(e) Recent Theory (model):
This recent theory is based on Lac Operon concept.
The gene for interferon contains three different regions i,o and Ig (structural gene for IFN). In normal state, i.e., absence of inducer, ‘i’ produces a repressor protein which blocks the operator and no transcription of Ig gene takes place (Fig. 21.29).
But when inducers come or infect the cells, they form a complex with the repressor protein formed by the ‘i’ gene. As a result of complex formation, the operator gene is now free from repression effect and the Ig structural gene transcribes to produce mRNA for interferon molecule. And it ultimately leads to the formation of interferon protein (Fig. 21.30).
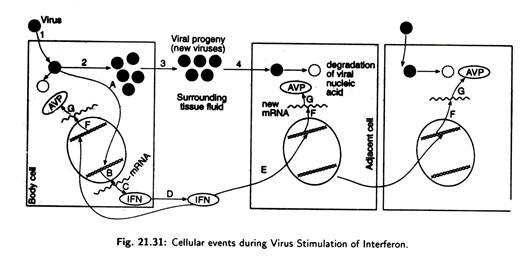
(f) Cellular events during virus stimulation of Interferon:
In Fig. 21.31 virus comes in contact with a body cell (1) and penetrates the cell membrane. The virus then releases its genetic material and multiplies (2). Released from the cell (3) into the surrounding tissue fluid, some of the new virus infects adjacent cells (4), and releases viral genetic material.
During early stages of infection of the first cell some events stimulates a gene in the cellular DNA which contained the stored genetic information for interferon (A). This leads to the production of mRNA for interferon.
mRNA leaves the cell nucleus (B) and is then translated by cell ribosome (C) into interferon protein. The newly produced IFN is secreted by the cell (D) into the surrounding fluid, where it contacts adjacent cell (E). The adjacent cell is activated by the IFN to produce new mRNAs (F) which are translated into proteins, (G) the antiviral proteins (AVP).
These proteins, in turn, can modify the cell protein synthesizing machinery and other biochemical events so that the viral nucleic acid functions poorly or is degraded— thereby inhibiting virus multiplication.
(g) Mechanism of Action of Interferon:
Interferon is the most potent known antiviral substance, being active in vitro at 10-12 to 10~14 moles/liter concentration. It is now known that interferon does not prevent the viruses to in fact the cells but rather inhibits its intracellular replication. It acts like a hormone and binds to a cell surface receptor and triggers the manufacture of some antiviral proteins (AVP), which then degrade the viral multiplication system.
The antiviral proteins (enzymes) are:
i. Protein Kinase
ii. Oligo Adenylate Synthetase
iii. Phosphodiesterase.
The protein kinase is synthesised in an inactive form and is activated by double-stranded (ds) RNA. This activated protein kinase phosphorylates a protein called eukaryotic initiation factor (eIF-2). This factor is essential for the initiation of protein synthesis. The phosphorylated eIF-2 can no longer bind to mRNA or ribosomes, as a result translation of mRNA into protein is blocked.
The oligoadenylate synthetase is inactive when synthesised and is also activated by ds. RNA. It then catalyses the synthesis of 2, 5, -oligoadenylic acid from ATP. This simple nucleic acid along with ds. RNA activates an RNA-endonuclease which is already present in the cell in an inactive form. The activated endonuclease degrade RNA and further hampers viral replication.
The third enzyme phosphodiesterase hydrolyses the terminal CCA group of tRNA making them non-functional. This leads to the fall in the concentration of functional tRNAs and brings the protein synthesis to a halt. The enzyme also can hydrolyze the oligoadenylic acid.
In its absence, the RNA-endonuclease reverts to its inactive state and mRNA degradation is terminated. This may serve to regulate the intracellular activity of the endonuclease and prevent a massive degradation of the cellular mRNAs.
It seems that initially interferon shuts off both viral and cellular protein synthesis, but the latter recovers after a few hours; may be due to the regulatory effect of the phosphodiestarase. Interferon also acts in a more subtle manner by impairing virus assembly and release and reducing the infectivity of the released particles. Interferon’s thus selectively inhibits viral replication and spares the host cell.
(h) Antitumor action of interferon:
Interferon inhibits multiplication of both cancerous and normal cells in vitro but its inhibitory action is more prominent on the rapidly dividing cells. Prom the recent years study it is evident that IFN kills cancer cells by enhancing the host immune response. There is strong evidence that interferon particularly IFN markedly enhances NK (Natural killer) cells activity.
This may occur in two ways:
i. By activation of pre-existing NK-cells.
ii. By triggering the differentiation of precursor cells into effective NK-cells.
The activated NK-cells themselves produce more interferon, which, in turn, further augments NK cell activity thus setting up a positive feedback circuit (Fig. 21.33). This results in a rapid amplification of NK cell activity.
(i) Therapeutic Uses of Interferon:
Few years back, the scarcity, impurity and enormous cost of human IFN had severely limited the scope of clinical trials in patients but advances in production and purification have made larger clinical studies possible. Interferon has been found to be of benefit in a large number of viral diseases. It is much more effective in the prevention of viral disease than their treatment.
a. The most comprehensive study of the antiviral action of IFN has been done in patients of Herpes Zoster (a disease). IFN treatment led to fewer herpetic vesicles, less acute pain, diminished severity of post herpetic complications as compared to control.
b. IFN α has been used for over a decade as an intranasal spray in the former USSR for the prevention of cold, influenza and other respiratory viral diseases.
c. Cancer therapy:
IFN represents a new approach to cancer therapy. It acts by enhancing the immuno-response of the organism and the side effects are minimum. Its complete lack of toxicity gives it a high therapeutic index.
The largest clinical trial with IFN was conducted by Hass Strander in Stockholm (1974) on the patients with Osteosarcoma. Treatment with IFN resulted in 50% fewer death and less recurrences of cancer as compared to untreated group. IFN is also effective in the treatment of breast cancer, Non-Hodgkin’s lymphoma, multiple myeloma and Leukemia.
Recent advances about interferon study:
Few years ago, use of interferon was beyond our reach:
i. With the availability of monoclonal antibodies to the interferon’s, purification has been simplified and the overall yield production increase. Since the IFN are hydrophobic proteins, reverse phase NPLC has been used effectively to purify interferon’s.
ii. The IFN mRNA has been isolated from Leukocytes, converted into C-DNA by reverse transcriptase and cloned via a plasmid in E. coli.
iii. The structure and base sequence of IFN gene has been determined.
iv. Small quantities of IFN produced in E. coli has been shown to be active in vivo and in vitro.
v. Amino acid sequences has been determined from its DNA sequence.
vi. In 1980, Biogen S. A. produced the first recombinant DNA induced interferon, one of which was undergoing clinical tests.
ii. Interleukins:
Interleukins can be defined as a group of signalling molecules produced by leucocytes which act on leucocytes. Some of these molecules are also produced by non-leucocytes and also acts on non-leucocytes. The interleukins which are produced by lymphocytes (T-cells) are often called lymphokines.
There are nine classes of interleukins:
(a) Interleukin 1 (IL1):
These are generated by monocytes, dendritic cells, some B cells, fibroblasts, epithelial cells, endothelium, astrocytes and macrophages. IL1 is divided into two subclasses such as IL1α (Mol. wt. 33,000) and IL1β (Mol. wt. 17,500).
The main target cells of IL1 are thymocytes, neutrophils, T and B cells, tissue cells. IL1 is mainly produced in response to damage, infection or antigens. It influences many cells and processes, stimulates T and B-cells, and induces inflammatory responses such as the production of postaglandins and degradative enzymes, viz., collagenase.
This is believed to be of special importance for the destruction of cartilage and bone. On many other cells IL1 induces the production of other cytokines which in turn have a secondary effect on skin cells. The classical effect of IL1 is as a pyrogen which accounts for its effect in the brain.
It,travels to the brain where it induces fever and acts to increase corticosteroid release. In the liver it induces the production of acute phase proteins which is liberated in response to injury.
(b) Interleukin 2 (IL2):
The main cell sources of IL2 are T-cells and NK cells. It acts on T-cells, B-cells and monocytes where it is the most powerful growth factor and activator. IL 2 also potentiates B- cell growth. The activation of monocytes and natural killer cells is important in amplifying the immune response.
IL2 is used in cancer therapy, particularly for patients suffering from retinal cell carcinoma. It may be related to the activation of lymphokine activated killer (LAK) cells which produce a cytotoxic, anti- canter effect.
(c) Interleukin 3 (IL3):
It is produced by T cell. The molecular weight of IL3 is 15,000. It stimulates the growth of precursor cells of red cells, granulocytes, macrophages and probably lymphocytes.
(d) Interleukin 4 (IL4):
It is produced by T-cell IL4 acts on B-cells to induce activation and differentiation leading in particular to production, of- IgG1 and IgE. It stimulates the growth of T-cells and also acts on macrophages to induce MHC class II expression. Excess IL4 play a part in allergic disease.
(e) Interleukin 5 (IL5):
This is generated by T-cells. IL5 is composed of 153 amino acids. In human it acts as a growth and activation factor for eosinophil’s whereas in mouse it stimulates B-cell to induce growth and differentiation.
(f) Interleukin 6 (IL6):
It is produced by macrophages, T-cells, fibroblasts and some B-cells. . IL6 acts on T- cell, B-cells, thymocytes and hepatocytes. The activity of IL6 includes the induction of differentiation of B-cells into antibody forming cell, production of acute phase proteins in the liver, etc.
(g) Interleukin 7 (IL7):
IL7 is produced by thymic stroma and acts on thymocytes. It is a T-cell growth and activation factor and a macrophage activation factor.
(h) Interleukin 8 (IL8):
This is produced by macrophages and T-cells. IL8 is involved in inflammation and cell migration. It is a powerful inducer of neutrophil and T-cell chemo taxis.
(i) Interleukin 10 (IL10):
It is also known as cytokine synthesis inhibitory factor which inhibits the production of interferon y and inhibits antigen presentation and macrophage production of IL1. IL6 along with TNq plays a role in the regulation of IgE.
(j) Colony Stimulating Factor (CSF):
The CSFs or colony stimulating factors induce the division and differentiation of bone marrow stem cells and the precursors of the blood leucocytes. Some CSFs also stimulate further differentiation of cells outside the bone marrow.
(k) Tumour Necrosis Factors (TNF):
The TNFs or tumour necrosis factors belong to a small group cytokine. There are two main types of TNF—TNFα and TNFβ. They are generated by macrophages and lymphocytes. The main target cells of TNFs are fibroblasts and endothelium. TNFβs have a variety of functions. They are particularly important in mediating inflammation, fibrosis, production of other cytokines, etc.
Type # 6. Complement:
The complement system is composed of a group of serum proteins that play a major role in the animal immune response. Thus complement protein can lyse antibody coated eukaryotic cells and bacteria. Complement can mediate inflammation (infected swelling) and attract active phagocytic cells. In general, complement proteins amplify the effect of antibodies.
The complement system is composed of at least 17 complement proteins. It acts in a cascade-like fashion, the’ activation of one complement protein constitute much of the globulin fraction of serum within plasma and other body fluid complement proteins are in an inactive state.
They are activated after the binding of antigen to the antibodies. Because complement activation involves binding of the protein complements to antigens-antibody complexes and to each other with their consequent removal from serum, the event is called complement fixation.
There are two pathways of complement activation:
i. The Classical Pathway: Activation of this pathway is initiated by the interaction of antibodies with an antigen that is usually cell-bound. Complement proteins interact here to form a membrane attach complex that creates a pore in the plasma membrane to the target cell.
If the target is eukaryotic cell, Na+ and H20 enter through the pore causing osmolysis of the cell. If the target is a gram-negative bacterium, lysozyme from the blood enters through the pore to lyse the cell.
ii. The Alternative Pathway: Activation of the alternative pathway does not require the binding of antibodies to antigen. Instead it plays an important role in the nonspecific immune defence against intravascular invasion by bacteria and some fungi, before the development of specific antibodies takes place.
Role of Complement Pathway in Evading Disease:
Complement activation acts as an integrated system during an animal’s defensive effort. Gram-negative bacteria are living at a local tissue site interact with the complements of the alternative pathway resulting in the generation of the biologically active fragments, opsonisation of the bacteria and, finally, the lytic sequence.
If the bacteria persist or invade the animal a second time, antibody response will activate the classical pathway which is much more rapid and efficient in mediating opsonisation and complement fragment generation.
Generation of certain complement fragments lead to important inflammatory effects. Mast cells release their contents and blood vessels. Also release of neutrophils from the bone marrow into the circulation is effected by these fragments.
Neutrophils then make their way to the site of the hyperemia where, in presence of another fragment, they attach the endothelium and leave the blood vessels. Thus, a directed chemotactic migration of neutrophils to the site of complement activation occurs.
An increased synthesis of complement fragments occurs to interact with the bacteria. All these events promote ingestion and ultimate destruction of the bacteria by neutrophils and macrophages.
Type # 7. T-Cell Receptor:
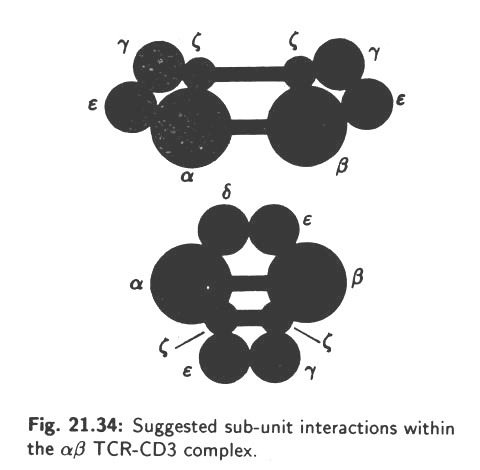
Antigen recognition by T-lymphocytes mediates through a specific receptor on T-cells. This is called TCR or T-cell receptor. TCR is a heterodimer consisting of an α chain and a β chain. Hence, TCR is actually known as α β TCR. The α and β chains are linked by disulphide bond. Later, a second TCR called yδ was discovered.
The α β and yδ forms of TCR are both combined physically with a series of polypeptides which are collectively called CD3. This association is necessary for surface expression of the TCR complex at the T-cell surface. The CD3 components show no amino acid diversity on different T-cells.
CD3 comprises at least 5 invariant polypeptides called y,δ, ɛ,ʗ, and n. The CD3, y, δ and e chains comprise an external immunoglobuhn like domain, a trans membrane segment containing a negative charged amino acid and a cytoplasmic tail-the CD3 ʗ and n) chains exist as disulphide-linked dimers.
Three dimeric forms exist such as ʗ-ʗ, n–n and ʗ – n. During T-cell activation, various components of the CD3 complex become phosphorylated on their intracytoplasmic portions.
The α β TCR consists of a disulphide-linked heterodimer of α and β polypeptide sub-units. Each polypeptide chain is made of two external immunoglobulin-like domains (110 amino acids) attached with the plasma membrane by a trans membrane peptide and a short cytoplasmic tail. The trans membrane region of both α and β chain contains one or two positively charged residue.
The yδ TCR is also made of y and δ polypeptide chain, i.e., heterodimer. The yδ chains can take several forms. The form containing a Cy1 constant region contains an inter-chain disulphide bond, whereas Cy2 forms are not disulphide linked. Duplication and triplication of a section of the y chain generates different forms of the y-protein.
The precise way in which the various immunoglobulin-like extracellular domains of the TCR (αβ or yδ) and CD3 (y, δ and ɛ) polypeptides associate is unknown. However, various models have been proposed. Two alternatives are shown in Fig. 21.34. The α,β and CD3c components form disulphide pairs.
Other, interactions are based on non-covalent bonds. The TCR complex may form higher order structures in the plasma membrane.
The T-cell receptors are encoded by L – V, D, J and C gene segments just like antibody chains and exhibit a great amount of diversity. This diversity is generated by genome rearrangements during T-lymphocyte differentiation from germ line cells.
The distribution of two forms of TCR is quite distinct in their anatomical location. The αβ TCR is present on more then 95% of peripheral T-cells whereas yδ TCR forms only minor proportion and is abundant in various epithelia such as intestinal epithelium, uterus and tongue.
Type # 8. Major Histocompatibility Complex (MHC) or Human Leukocyte Antigen (HLA):
The ability of the vertebrate immune system to distinguish self from non-self depends largely on a group of membrane protein known as the major histo-compability complex or MHC. In humans, the MHC is called the HLA (Human Leucocyte Antigen) group. These markers are present on the surface of most cells and are slightly different in each individual. A group of closely linked genes codes for MHC proteins.
These genes are polymorphic, i.e., within the population there are multiple alleles for each locus (sometimes more than 40 alleles for a given gene). With so many possible combinations, no two people except identical twins are likely to have the same HLA protein on their cells. Thus the HLA gives a biochemical fingerprint. The more closely related two individuals are, the more HLA group they have in common.
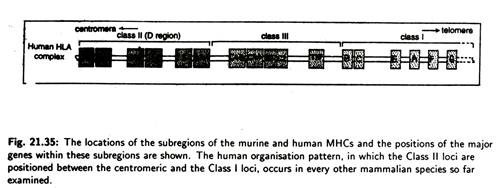
The MHC is divided into three groups of genes (Fig. 21.35) that code for distinct sets of proteins. These proteins differ in terms of tissue distribution and chemical structure.
Three groups of genes of human MHC are designated as class I, class II and class III loci and their corresponding encoded membrane protein are designated as class I, class II and class III antigen molecules. MHC class I molecules are found on most nucleated cells and are important in distinguishing between self and non-self.
They bind with foreign antigens forming a molecular complex that is displayed on the cell surface and activates the other cells of immune system (Fig. 21.36). For example, suppose the macrophage engulfs a bacterium, breaks it down and presents bacterial antigens on its surface in combination with MHC.
Helper T cells are activated when the receptors combine with this foreign antigen MHC complex and they are stimulated by interleukins (a group of molecules involved in signalling between cells of the immune system) secreted by the macrophages.
B-cells can combine with specific antigens. However, B-cells are generally activated by interleukins secreted by activated helper T-cells. Once activated the B-cell divides, forming a clone of cells. Some of these differentiate into plasma cells that secretes antibodies. Antibodies then neutralize the bacteria by binding directly to their surface antigens and prevent them from attacking other cells.
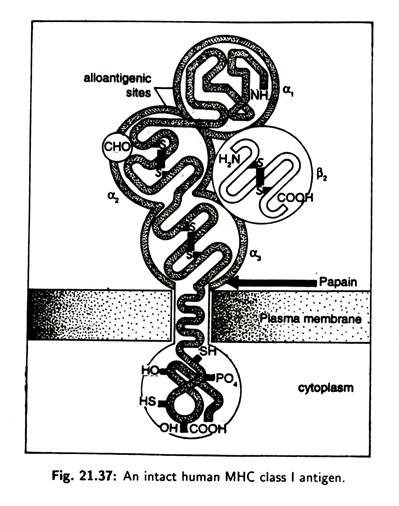
The structure of MHC class I molecules comprises of a glycosylated heavy chain, non-covalently associated with β2 micro-globulin (Fig. 21.37), a polypeptide which is also found free in serum. The heavy chain of class I molecule consists of three extracellular domains— α2, α 2, α 3—and a trans membrane (across the plasma membrane) region and a cytoplasmic tail.
The α 3 domain is closely associated with the non-MHC encoded peptide, β2 micro-globulin which is stabilized by an intrachain disulphide bond. Alloantigenic sites (carrying determinants specific to each individual) occur on the α1 and α 2 domains and there is a carbohydrate unit attached to the α 2 domain (CH0). Papain cleaves the molecule close to the outer margin of the plasma membrane.
MHC class II antigens are found particularly on B-cells, macrophages, some T-cells and dendritic cells (specialised cells located throughout the body, especially in the spleen and lymph nodes). Class II antigens regulate the interactions among T-cells, B-cells and antigen- presenting cells.
MHC class II antigens bind with peptides from proteins that have entered the cell via foreign sources such as bacteria. The MHC class II and foreign antigen complex is displayed on the cell surface and stimulates helper T-cells.
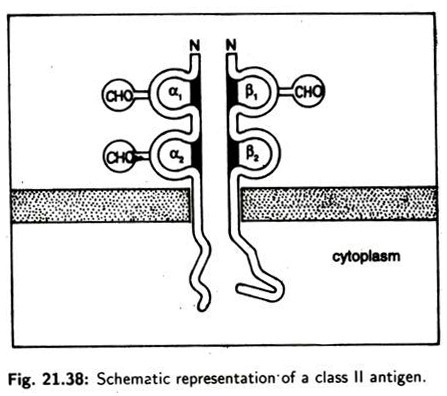
The structure of class II molecule (Fig. 21.38) consists of two non-identical peptides (a and P) non-covalently bound which traverse the plasma membrane. Both chains have two globular domains outside the cell. The domain closest to the membrane in each chain is structurally similar to immunoglobin domains.
All domains (α, α 2, β and β2) are stabilized by inter-chain disulphide bonds. Both chains have carbohydrate units attached. β -chain contains the alloantigenic sites. The class III region of the chromosome contains a rather diverse collection of 20 genes including those encoding the components of the alternative complement activation pathway.
There are no established functional or structural similarities between class III gene product and the class J or class II molecules.
Type # 9. Adjuvant:
The word adjuvant is derived from the Latin ad (to) + juvare (to help) which means to help. It refers to the fact that when soluble antigens are given experimentally, it helps to make stronger T and B-cell mediated responses if they are mixed with bacterial components.
Bacterial cell wall components such as lipopolysaccharide, glycolipids, a rabino galactan, peptidoglycan have adjuvant properties that is they are recognised by the immune system as a non-specific signal that, boosts immune activity.
The best known adjuvant in laboratory use known as complete Freund’s adjuvant consists of killed Mycobacterium tuberculosis suspended in oil which is then emulsified with the aqueous antigen solution.
This effect possibly reflects the fact that when the antigen-specific immune response is evolved, it did so in a tissue environment already containing these pharmacologically active bacterial components. The response to a pure bacterial antigen injected without adjuvant active bacterial components can be defined as an artificial situation that is not found.
Type # 10. Autoimmunity:
Autoimmunity is a form of hypersensitivity that occurs when the body reacts immunologically against its own tissues. Some of the diseases that result from such failure in recognising self (self-tolerance) are rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosis (SLE), insulin-dependent diabetes, psoriasis and scleroderma.
In multiple sclerosis and other autoimmune diseases the body produces abnormal antibodies. Genetic factors are known to play a role. In multiple sclerosis, antibodies attack the glial cells that are produced in myelin sheath surrounding the neurons in the brain and spinal cord. Patients generally suffer weakness and visual problems and become progressively more disabled.
Studies also indicate that viral or bacterial infection often precedes the onset of an autoimmune disease. Some pathogens have evolved a tactic known as molecular mimicry. They trick the body by producing molecules that look like self-molecules.
For example, an adenovirus that causes respiratory and intestinal illness produces a peptide that mimics myelin protein. When the body launches responses to adenovirus peptide, it may also begin to attack the similar self-molecule, myelin.
Autoimmunity may be developed in many ways:
i. If the self-antigens undergo alternation in their chemical or physical form.
ii. Introduction of foreign antigens into the body which are closely related to self antigens. In such cases autoimmunity may arise by producing cross-reacting antibodies that trigger B-cells directly.
Type # 11. Hypersensitivity or Allergy:
Hypersensitivity or allergy is an exaggerated immune response that results in tissue damage and is shown when the individual is on a second or subsequent contact with the antigen. Hypersensitivity reactions may be immediate when the reactions are slow. The major difference between the two is the nature of immune response to the antigen.
In allergic reactions, individuals manufacture antibodies against mild antigens, called allergens, that do not stimulate a response in non-allergic individuals. Allergic reactions are referred to as Type I hypersensitivity (See below). In many cases of allergic reactions, distinctive IgE immunoglobulin’s are produced.
Let us examine a common allergic reaction— a hay fever response to pollen allergy (Fig. 21.39). The first step is sensitisation. Macrophages destroy the allergen and display fragments of it to T-cells. The activated T-cells then stimulate B-cell to become plasma cell and produce IgE.
These antibodies attach to receptors on mast cells which axe large connective tissue cells filled with distinctive granules. Each IgE molecule attaches to a receptor by its C region end leaving the V region end free to combine with the pollen allergens.
The second step is activation of mast cells. When a sensitized allergic person inhales the microscopic pollen, allergen molecules rapidly attach to the IgE on mast cells. The binding of allergen with IgE antibody stimulates the mast cells to release granules filled with histamine and serotonin that cause inflammation.
These substances cause blood vessels to dilate and capillaries to become more permeable leading to edema and redness. Such responses cause the victim’s nasal passages to become swollen and irritated. Their nose run, they sneeze, their eye water and they feel generally uncomfortable.
A third step may take place in which the allergic response is prolonged. Chemical compound released by the mast cells entice certain white blood cells to leave the circulation and migrate to the inflamed area. These cells then secrete compounds that damage tissue and prolong the allergic reaction.
In allergic asthma, an allergen response occurs in the bronchioles of the lung. Mast cells release Slow Reacting Substance of Anaphylaxis (SRS-A) which cause smooth muscle to constrict. The airways in the lungs sometimes contract for several hours making breathing trouble. Peter Gell and Robert Coombs (1963) classified reactions responsible for hypersensitivity into 4 types—I, II, III and IV.
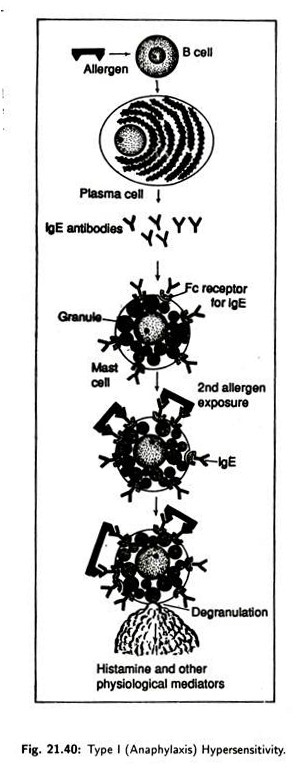
(i) Type I (anaphylaxis) Hypersensitivity:
Such hypersensitivity is characterised by an allergic reaction occurring immediately following an individual’s second contact with the responsible antigen (allergen). Upon initial exposure to an allergen, B-cells axe stimulated to differentiate into plasma cells (Fig. 21.40) and produce specific IgE, which, after synthesis, bind to Fc receptors of mast cell basophils and sensitizes these cells causing allergy.
When second exposure to the allergen occurs, the allergen attaches to the surface bound IgE on the sensitized mast cells causing degranulation. Degranulation releases physiological mediators like histamine, heparin, proteolytic enzymes etc. which trigger smooth muscle contraction, vasodilatation, increased vascular permeability and mucous secretions.
Anaphylaxis may be:
(a) Systematic Anaphylaxis:
It is generalized response occurring when an individual sensitized to an allergen receives a subsequent exposure to it. The reaction shown is immediate due to large amount of mast cell mediators released over a short period of time.
Respiratory impairment is caused due to smooth muscle contraction in the bronchioles, arterioles dilate, causing low blood pressure, increased capillary permeability is seen, and the individual may die in a short time due to reduced venous return and reduced blood pressure. Examples of such allergens are penicillin, insect venom etc.
(b) Localised Anaphylaxis:
Symptoms shown depend primarily on the route by which the allergen enters the body. An example of localised anaphylaxis is Hay fever. Initial exposure to the allergen is caused by airborne substances like plant pollen, fungal spores etc., and there sensitize mast cells located in the mucous membranes.
Re-exposure to the allergen causes local anaphylaxis manifested as itchy and tearing eyes, congested nasal passages, coughing, sneezing, etc.
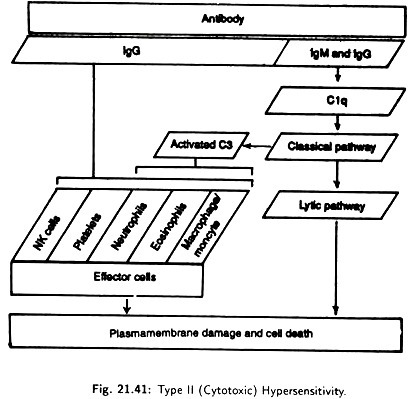
(ii) Type II (cytotoxic) Hypersensitivity:
Type II (Cytotoxic) hypersensitivity (Fig. 21.41) is generally called a cytolytic or cytotoxic reaction because it results on the destruction of host cells, either by lysis or toxic substance. IgG or IgM antibodies are directed against cell surface or tissue antigens. They react with complement and effector cells through their Fc region.
The best example of such hypersensitivity is seen when a person receives a transfusion of blood from a donor with a different blood group.
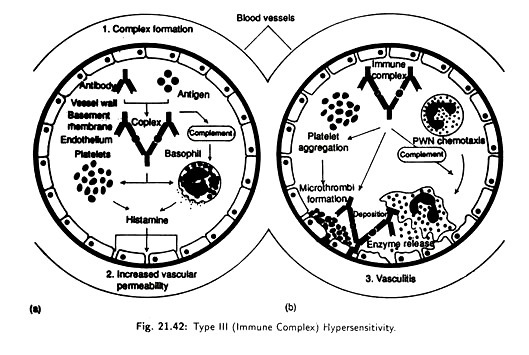
(iii) Type III (immune complex) Hypersensitivity [Fig. 21.42]:
This involves the formation of immune complexes. Normally these complexes are removed effecting by the monocytes of the reticuloendothelial system. But in the presence of excess amount of some antigens, the antigen-antibody complex may not be effectively removed.
Their accumulation can lead to a hypersensitivity reaction that triggers a variety of inflammatory process, especially causing damage to blood vessels, kidney glomerular basement membranes and to the skin.
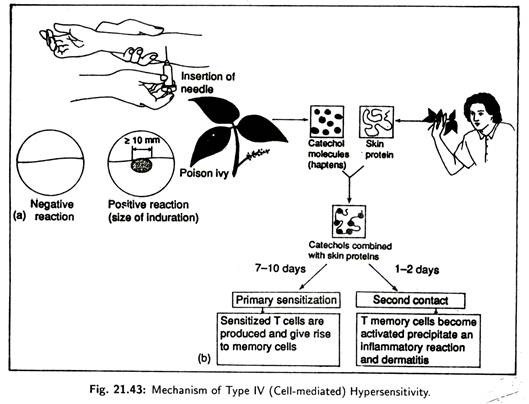
(iv) Type IV (cell mediated) Hypersensitivity [Fig. 21.43]:
Here delayed T-cells mediated immune reactions are involved. A major factor here is the time required for a special type of T-cells called delayed type-hyper sensitivity T(TDTH) cells to migrate and accumulate near the antigens. This usually takes a day or more.
Type IV reactions occur when antigen, specially those binding to tissue cells, are phagocytosed by macrophages and then presented to the cell TDTH surface. Contact between antigen and TDTH causes the cell to proliferate and release lymphokines. Lymphokines attract lymphocytes, macrophages and basophils to the affected tissue causing skin damage.
Examples of Type IV hypersensitivities include allergic contact dermatitis caused by haptens that combine with the proteins in the skin, attracting the immune response. Haptens act as antigenic determinants and skin haptens are cosmetics, plant materials, metal, jewellery etc.
Type # 12. Immunological Memory:
The immune system has the ability to learn and it has a memory. The first exposure of B-cell to an antigen leads to a slowly rising synthesis of antibody particularly IgM. This is known as the primary immune response. A second encounter with the same antigen leads to a more rapid and greater response chiefly IgG production. This is known as the secondary immune response.
Only a primary response provokes the secondary response to provide immediate protection whenever the same antigen intrudes again. Measles, mumps, chicken pox and many more diseases are suffered only once during a lifetime.
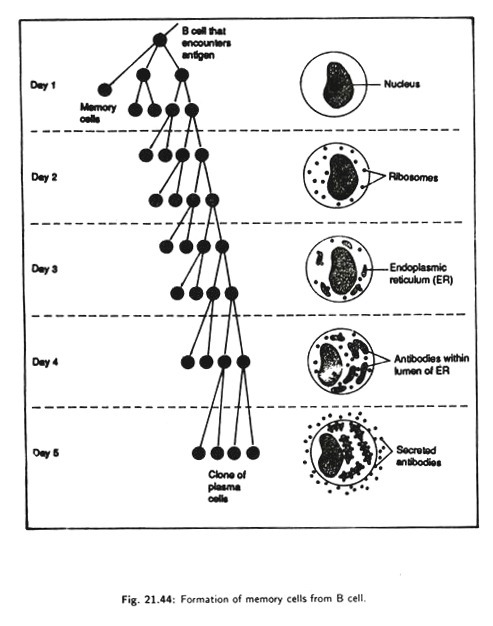
The first encounter with the intruder leaves survivors immune to subsequent infection. How does the secondary response occur? How is the immune system able to learn to recognize the. antigen to which it was previously encountered or exposed? The basis of learning in the immune system is the formation of long-lived memory cell from the B-cells during the primary response.
After encounter with first antigen and stimulation by factors produced by helper T-cells, B-cells starts to divide and produces a number of cells. Some cells differentiate into memory cells (Fig. 21.44) and the remaining cells differentiate into antibody producing cells.
The memory cells persist in the circulation for the life of the organism even without further antigenic stimulation. Although antibodies and plasma cells are following removal of the antigenic stimulus, memory cell persist for years providing immunological recall.
The circulating cells each carry on their surface the particular immunoglobulin (IgG or IgA) that will bind perfectly to a particular reinvading antigen. Immunity, therefore, is due to the presence of antigen specific memory cells that launch a swift secondary immune response whenever the antigen is encountered.
This type of protection is called active immunity. Antigen-specific active immunity can be safely acquired by using vaccines which are some of the most potent weapons against diseases. Vaccines are modified forms of disease-causing microbes; these variants have lost the ability to cause disease but retain the same antigens as their dangerous counterparts.
An immune system exposed to a vaccine (and usually several booster doses) builds memory cells without the danger of disease developing during the vulnerable primary response. In this way we can now safely promote active immunity against polio, measles, mumps, diphtheria, tetanus, rabies and many other dangerous diseases.