ADVERTISEMENTS:
Reserve materials are the food matters of the plants, and so they are directly concerned with the nutrition of the plants.
The three types of reserve materials are: (1) Carbohydrates (2) Nitrogenous Matters and (3) Fats and Oils.
Type # 1. Carbohydrates:
These are chemically composed of carbon, hydrogen and oxygen, hydrogen and oxygen always occurring in the same proportion as in water. So in all carbohydrates hydrogen-oxygen ratio would be two: one. Of many carbohydrate food matters, some are simple and soluble in water, whereas others are complex and insoluble.
ADVERTISEMENTS:
On heating they lose water and a black mass is left behind, which is nothing but carbon. Carbohydrates are very important energy-giving food, present more abundantly in the storage regions.
An account of the common carbohydrate food matters is given below:
1. Sugars:
These are soluble carbohydrate food matters, usually sweet to taste. They occur as reserve materials in many monocotyledons and also in some dicotyledons like beet and carrot. Many of them are strong reducing agents. The common sugars of the plants have the formula, C6H12 O6. They are called hexoses, as six carbon atoms are present. Pentoses or sugars with five carbon atoms are not common in plants.
Those having one basic sugar group are referred to as monosaccharides. Monosaccharides like glucose and fructose, particularly the former, are abundant in all plants.
ADVERTISEMENTS:
Glucose, C6H12O6, is really the basic material of metabolism, being the first stable product of photosynthesis. It is synthesised by the chloroplasts out of raw materials, water and carbon dioxide gas in presence of sunlight. So it is present in all green plants.
Fructose or fruit sugar with the same formula, C6H12O6, though differing in the structure of molecules, occurs m the fruits. Two other common hexose sugars of plants are mannose and galactose.
Sucrose or cane sugar is a disaccharide, having two of the sugar groups, with the formula C2H22O11. It is our table-sugar. It is widely distributed in some plant tissues, often reaching very high concentration in storage organs.
Sucrose is quite valuable commercially and is extracted mainly from sugar-cane stems and beet roots. On hydrolysis— breaking down by chemical addition of water, it yields one molecule of glucose and one molecule of fructose.
Maltose is another disaccharide sugar commonly found in the germinating seeds during digestion of starch. Ityields two molecules of glucose on hydrolysis.
The most widespread trisaccharide in plants is raffinose, which occurs in very small quantities. On hydrolysis it yields three molecules of hexoses—glucose, fructose, and galactose.
Tests:
Sugar solution is taken in a test-tube. Equal quantities of Fehling’s solutions I and II are added and boiled. Orange-red precipitate of cuprous oxide indicates the presence of a reducing sugar. To sucrose solution add a little sulphuric acid and boil gently. It is hydrolysed to glucose and fructose. Now it would give Fehling’s reaction.
2. Inulin:
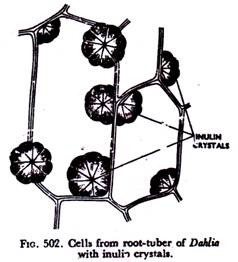
It is a polysaccharide carbohydrate which forms a powder-like compound and occurs in colloidal condition in the cell-sap of the vacuoles of plants like Dahlia, Artichoke—of the family Compositae. It is by no means of common occurrence in plants.
ADVERTISEMENTS:
Inulin may be precipitated by treatment with alcohol or glycerine. In fact, when blocks of root tubers of Dahlia are allowed to remain in alcohol or glycerine for some time and sections are examined under the microscope, beautiful fan-shaped crystals (Fig. 502) are noticed in the cells.
On hydrolysis inulin yields only fructose. Inulin solution turns yellowish brown when treated with acid phloroglucin. On precipitation the crystals are easily recognised.
3. Starch Grains:
Starch grains are the complex insoluble carbohydrates. They are the most important carbohydrate reserve materials of the plants and are of universal occurrence in the green plants with the exception of some algae.
ADVERTISEMENTS:
In some monocotyledons also the carbohydrate reserve is in form of sugars. Starch grains may occur in all parts of the plants, but they are more abundant in the storage organs, cereals, fruits and seeds.
They are very complex chemically with the formula, (C6H10Os)n, where the value of n is not clearly known. It is formed out of unknown number of hexose groups and is thus a polysaccharide. For utilisation by the plants starch is ultimately converted into glucose by enzyme actions.
There are two types of starch grains: assimilatory starch and reserve starch. Assimilatory starch grains are temporary bodies formed by the chloroplasts in day-time during manufacture of glucose. As glucose is soluble in water the cell-sap becomes more and more concentrated with increasing manufacture of glucose, so much so that the saturation point is reached soon.
As further manufacture would not be possible then due to physical reasons, the chloroplasts convert glucose into insoluble starch, which are called assimilatory starch. They are granular in appearance and temporary in duration, as they are reconverted into sugar with nightfall.
ADVERTISEMENTS:
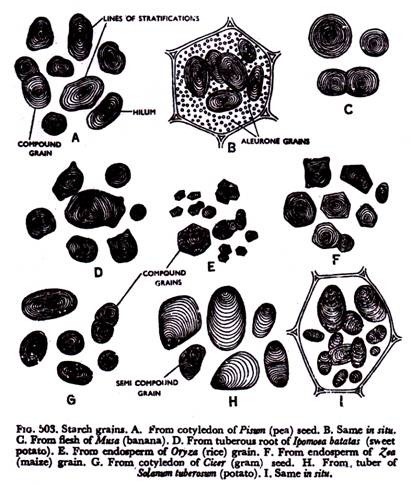
Surplus of sugar is converted into permanent starch grains to be stored up for future use by the colourless leucoplasts—the amyloplasts. These are reserve starch (Fig. 503). They occur profusely in storage organs like roots and underground stems and in seed and fruits.
They are of diverse size and shape. Some of them are large enough to be seen even with the naked eye, whereas most of them are microscopic. Every starch grain has a shiny refractive point, known as hilum, which is the starting point.
Starchy materials are deposited in layers around the hilum, ultimately giving the grain a distinct stratified appearance. The layers are referred to as lines of stratifications. The stratified nature of the grain is mainly due to the fact that the layers deposited in light and darkness differ in water content and density as a result of diurnal periodicity of the action of the plastids.
The innermost layer is particularly rich in water which explains the cracks frequently noticed in desiccated starch. The alternate layering’s become quite clear in the photographs taken in polarized light. Starch grains are regarded as sphaero-crystals consisting of layers of C9K10Os units arranged in a regular ‘space-lattice’.
ADVERTISEMENTS:
In a starch of potato tuber the hilum is located at one end of the grain, the deposition of layers being unequal. These are called eccntric grains, whereas in grains occurring in pulses, the hilum is more or less at the centre due to uniform deposition of the layers.
These are concentric grains. A grain having one hilum and its surrounding lines of stratifications is known as simple. More than one grain may be adpressed together making it compound.
In rice and other cereals, potatoes, etc., compound grains are of common occurrence. A peculiar type of grain, referred to as semi-compound or half-compound is also found. In that case there are two hila with their lines of stratifications, which are ultimately bounded by common lines of stratifications.
Starch has two components, viz., amylose and amylopectin, which differ in physical properties. Amylose is more water-soluble and turns deep-blue with iodine solution, whereas amylopectin is more waxy in nature and gives light blue-violet coloration with iodine solution.
Iodine reaction is, at any rate, the most characteristic test of starch. Starch is synthesised by the plastids out of glucose; and on complete hydrolysis it yields glucose.
Starch grains vary considerably in size and shape. They are usually oval in potato varying from 70 to 100 micra m diameter, 30-45µm in wheat, 12 to 18µm in maize. But the shape is more or less constant in a particular type.
ADVERTISEMENTS:
That is how the plant source of starch in a given sample of flour can be determined. The percentage of starch is fairly high in storage organs, it is 15-30% of dry weight in potato, even 50 to 70% of dry weight in the cereals.
4. Glycogen:
It is another insoluble polysaccharide with the formula (C6H10O6)n. As it is abundantly present in the muscles and liver of the animals, it is also called animal starch, though the term is not a happy one.
Glycogen occurs in blue-green algae, slime moulds, fungi and bacetria and serves as reserve food. It is ultimately broken down to glucose by enzyme action and utilised as such. It gives reddish coloration with iodine solution.
5. Hemicellulose:
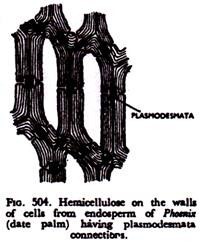
Cellulose (C6H10O5)n, is another polysaccharide carbohydrate which is much more complex than starch. It is particularly valuable, because the cell wall is primarily composed of this substance. Cellulose is not only insoluble, but it is also indigestible.
So it is seldom used as food. In the seeds of plants like date palm, ivory nut palm another insuluble carbohydrate resembling cellulose is deposited as extra layers making the walls very thick and hard (Fig. 504).
ADVERTISEMENTS:
This is reserve cellulose or hemicellulose. Though insoluble in water, it readily dissolves in alkalies. Hemicellulose is utilised as food by proper enzyme action.
6. Pectins:
Pectins, also called fruit jellies, are present in many plants, particularly in the fruits. They are readily soluble in water. Apart from being used as food by the plants and animals, pectins can increase the water-holding capacity of the cells.
The cementing material between two adjacently-lying cells, the middle lamella, is made of pectin compound. They are abundantly used in the preparation of jams and jellies.
7. Gums and Mucilages:
Gums and mucilages are widely distributed in the plants. Though they do not dissolve in water, they form a slimy mass when moistened. Besides their utility as reserve food, they help the plants in diverse ways like holding water, checking rapid loss of water and in facilitating dispersal of seeds. To human beings they are particularly useful in industries and medicine.
Type # 2. Nitrogenous Matters:
Nitrogenous matters, commonly called proteins, are the most complex organic food matters of the plants. They are specially needed in the regions of active growth. Apart from their utility as very important tissue builders, they form an integral part of protoplasm itself, and so are present in every living cell. Chemically these extremely complex matters are composed of carbon, hydrogen, oxygen and nitrogen, and often sulphur and sometimes phosphorus.
Chlorophyll, the most essential green pigment of plants, is a magnesium-containing protein and haemoglobin of blood, the most efficient oxygen-carrier of our body is an iron-containing protein.
The nitrogenous matters may be put into two categories: simple soluble ones, called amino-acids, and complex insoluble ones occurring in form of grams, known as proteid grains.
It goes without saying that all proteid grains are formed out of amino acids, which have been very aptly called ‘building scones’ of proteins, through complicated chemical processes. In fact, all the steps in protein synthesis are not clearly known. The amino-acids are often found in colloidal solutions in water. Complex-proteins are broken down to amino-acids by proper enzyme action.
In that form they move to the different parts of the plants where they are needed and utilised. Commonly occurring amino- acids of plants are tyrosine, histidin, etc. Amides are salts of amino-acids, corresponding to inorganic salts; common examples are asparagine, glutamine.
The insoluble proteid grains are very complex. The formulae of a few common proteids would go to show how tremendously large the protein molecules are: gliadin, a protein of wheat: C636H1068N196O211S5; zein, a protein of corn: C736H1161N174O208S3.
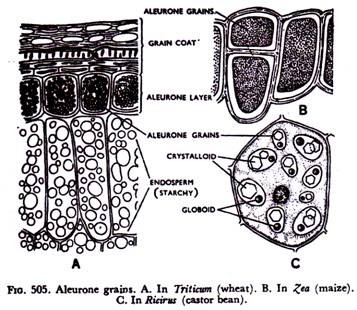
In the storage regions they occur as grains called aleurone grains. Pretty good number of plant proteins has been found occurring in crystalline or amorphous forms. On the average a plant protein is said to be composed of 51 % carbon, 25% oxygen, 7% hydrogen, 16% nitrogen, 0.4% sulphur and 0 4% phosphorus.
It has been said that most of the proteid grains stored as reserve food occur as small aleurone grains. In cereals a layer of cells just beneath the graincoat, composed of pericarp and testa, remains filled with aleurone grains (Fig. 505A).
This layer is referred to as aleurone layer. They are also present in maize (Fig. 505B) and other cereals. In pulses like pea (Fig. 503B) they remain as small grains mixed with comparatively larger starch grains.
More complex aleurone grains are found in the endosperm of castor-oil seed (Fig. 505C). Here the grain has a protein matrix in which occurs a large crystalline body, called ctystalloid, and a small round body, known as globoid.
Of the two, it is the crystalloid which is nitrogenous, the globoid, chemically, being a double phosphate of calcium and magnesium.
Test:
Two common microchemical tests for proteins are used, (i) A section or suspension of tissue in water is treated with strong nitric acid. A yellow coloration is noticed. Now a few drops of strong ammonium hydroxide are added. The colour changes to orange. This is xanthoprotein test.
(ii) One ml of 20% sodium lydroxide and one drop of 1% copper sulphate solution are added to the material. A violet colour is formed which indicates the presence of protein. This is Biuret test.
Type # 3. Fats and Oils:
Fats and oils are also very important energy giving reserve materials of plants. They are composed of carbon, hydrogen and oxygen, but not in the same proportions as in carbohydrates. They have much more of carbon and hydrogen and relatively very low oxygen. The formula of common fat, olein, present in olive oil—C5,H104,O4, is suggestive.
Due to occurrence of relatively low oxygen and high carbon and hydrogen fats and oils release much more energy than carbohydrates. Fats which are liquids at room temperature are called oils.
They are lighter than water, the specific gravity ranging from 0.875 to 0.970, and are insoluble in water. They dissolve in substances like chloroform, ether and acetone. Fats leave greasy spots on a piece of paper.
As oils they are probably present in all living cells, often in form of drops in the cytoplasm. These insoluble food matters are compounds (esters) of fatty acids and glycerine. Principal fatty acids are oleic acid, palmitic acid.
They are abundantly present in storage organs, in seeds, embryos and meristematic tissues. Fats are possibly derived directly from carbohydrates. This inference is based on the facts that during the maturation of oily seeds there is a decrease of carbohydrates and increase in fats, and also that during germination the process is reversed, fats being converted into sugars.
They are supposed to be formed directly by cytoplasm and also by special fat-forming plastids called elaioplasts. Fats usually occur in the plants as oils. These oils arc non-volatile and, hence are called fixed oils. They are generally extracted by pressure.
Some fatty oils are also used as medicines, e.g. chaulmoogra oil from Hydnocarpus Kurzii of family Flacourtiaceae in leprosy and croton oil from Croton tiglium of family Euphorbiaceae as one of the most strong purgatives.
Besides their utility as source of food, fats are also used in many industries. They saponify when treated with caustic soda or caustic potash. As a result of the said treatment soap is formed, which is nothing but a sodium or a potassium salt of a fatty acid.
Test:
Fats and oils stain red with alkalin and Sudan IV. When treated with 1% solution of osmic acid in water they turn black in colour.