ADVERTISEMENTS:
In this article we will discuss about Cellular Immune Response in Human Body:- 1. Subject-Matter of Immunity 2. Definition of Immunity 3. Types 4. Regulation 5. Immunoassay Techniques 6. Inflammation 7. Immunisation 8. Transplantation Rejection —An Immune Response.
Contents:
- Subject-Matter of Immunity
- Definition of Immunity
- Types of Immunity
- Regulation of Immune Responses
- Immunoassay Techniques
- Inflammation
- Immunisation
- Transplantation Rejection —An Immune Response
1. Subject-Matter of Immunity:
ADVERTISEMENTS:
Historical accounts of immunity go back as far as 430 BC. The Greek historian Thucydides in his book History of the Peloponnesian War mentioned a disease that severely affected the population of Athens. He remarked that the sick were cared by those who had recovered from such disease.
Athenians then understood that resistance against the disease was acquired by the people while recovering from such disease. During the following centuries it became well-established that recovery from diseases like small pox confers lifelong immunity to those diseases.
But the basis for immunity, however remained a mystery until 1890 when S. Kitasato and E. Von Behring published their studies on the tetanus toxin.
They reported that animals became immune to tetanus after they had been injected with a small amount of denatured tetanus toxin. They also reported that blood from those animals contained a substance capable of inactivating native toxin and that their serum, when injected to other animals, developed immunity to tetanus on the latter.
Our environment contains a great variety of infectious microbes—viruses, bacteria, fungi, protozoa and multicellular parasites. Animals as well as human beings always come in contact with such microbes, yet few of those contacts cause disease.
Maintenance of a relatively good health enjoyed by most individuals is dependent upon a complex set of defences against infection. These defence include physical barriers (i.e., exterior defences) to invasion by microorganisms (Fig. 21.1), anti-microbial chemicals and specialised host cell (Fig. 21.2) that eliminate most microorganism that invade body tissue.
An inborn or artificially acquired capacity is present in animals by virtue of which they can combat the threat imposed by pathogens. This capacity is termed immunity and the study of immunity is termed immunology. The immune system consists of specialised host cells (Fig. 21.2) which destroy or inactivate pathogens which, after invading the surface barrier, i.e., skin of the host, manage to enter the body.
2. Definition of Immunity:
The word immune comes from the Latin word immunis (from in – not + minus-serving) which means—free of burden. It refers to the general ability of a host to resist to a particular disease or diseases. Animals including human beings are continuously exposed to microorganisms, their metabolic products or other foreign macromolecules which are all capable of inducing disease.
The inborn or artificially acquired capacity of animals to ward off the potential threat of disease posed by the abovementioned factors is termed immunity.
3. Types of Immunity:
Immune response shown by the host is a specific and complex series of defensive actions. Any immune response involves, firstly, recognition of the pathogen or other foreign materials as not belonging to itself and, secondly, develops a specific immune response leading to the destruction or neutralisation of these substances.
ADVERTISEMENTS:
On the other hand, immunity signifies all properties of the host that confer resistance to a specific infectious agents.
Broadly speaking, different types of immune responses and immunity fall into two categories:
i. Innate or non-adaptive or natural immunity—the power of resistance may be inherited.
ii. Adaptive or acquired immunity—not natural but acquired as a result of previous infection or inoculation.
The important difference between these is that an adaptive immune response is highly specific for a particular pathogen. Moreover the response improves with each successive encounter with the same pathogen; in effect the adaptive immune system remembers the infectious agent and can prevent it from causing disease like measles, diphtheria etc.
Adaptive immune responses generate a lifelong immunity following infection. The two key features of the adaptive immune response are, thus, specificity and memory.
1. Natural Immunity:
It is naturally present by birth in an individual and is not acquired through previous contact with the pathogens. This is actually inherited resistance to infection and is concerned with species, races or individuals. The power of resistance to infection varies with different individuals of the same species, and with age also.
The naturally acquired immunity may be:
ADVERTISEMENTS:
i. Active
ii. Passive
i. Active:
Naturally acquired active immunity occurs when an individual’s immune system contacts an appropriate antigenic stimulus during normal daily activities. The immunity system responds by producing antibodies and sensitized lymphocytes that inactivate or destroy the antigen. The immune produced may either be lifelong, e.g., measles or chicken pox or last for only few years, e.g., for tetanus.
ADVERTISEMENTS:
ii. Passive:
ADVERTISEMENTS:
Naturally acquired passive immunity means transfer of antibodies from one host to another. For example, antibodies of a pregnant woman pass across the placenta to her fetus. If the mother is immune to disease like Polio, the placental transfer renders the newborn immunity to polio.
Certain other antibodies can pass through the first secretion (called colostrum) or the mammary glands. Naturally acquired passive immunity generally lasts only for a short period—for few weeks or months only.
2. Acquired Immunity:
Acquired immunity is not present by birth with an individual. This type of immunity is acquired during the lifetime of an individual as a result of previous infection.
It is basically of four types:
a. Active Acquired Immunity:
ADVERTISEMENTS:
Active acquired immunity is an adaptive resistance that is built up when an individual is immunized by a vaccine. A vaccine is nothing but a preparation of either killed microorganisms or inactivated microbial toxin.
These are injected or administered to induce immunity artificially—Active acquired immunity develops very slow in a number of days and weeks but persists for several years. The mechanism involves either humoral response or cell-mediated response.
Examples of some disease and their corresponding vaccine are in Table 21.1:
b. Humoral Immunity:
The term humor means a fluid and humoral immunity refers to the molecules solution in the body. This type of immunological reaction is mediated directly against the microorganism through the circulating blood proteins called antibodies. The antibodies are actively produced by B lymphocytes/plasma against cell antigens of microorganisms and their products (Fig. 21.3).
These antibodies may induce resistance by:
i. Blocking the infective capacity of microorganisms.
ii. Making microorganisms susceptible to phagocytic action.
iii. Neutralizing toxins or cellular enzymes.
iv. Killing bacteria or lysing with complement.
v. Combining with cellular antigens that interfere with phagocytosis.
c. Cell Mediated Immunity:
It is a complex response requiring immunologically specific and non-specific features. This response may or may not be accompanied by humoral antibody formation and the principal agent is an immunologically lymphoid cell (T lymphocytes).
These are specific circulating cells that recognize foreign materials and initiate a chain of reactions which include mononuclear inflammatory responses, cytotoxic destruction of invading cells, activation of phagocytic macrophages and delayed hypersensitivity in tissues.
d. Passive Immunity:
Artificially acquired passive immunity develops against an infective agent induced by the administration of antibody against that agent. The antibody is produced and derived from host and is introduced into another host. For example, antitoxin for Botulinum produced in a horse is given to a human suffering from botulism food poisoning.
Passive immunity lasts for a short time, usually a few weeks only. The antibody molecules after entering into host constantly breakdown without forming new ones. That is why this type of immune response shows immediately.
4. Regulation of Immune Responses:
The immune response is regulated by a variety of control mechanisms which help to restore the immune system to a resting stage when reaction to a given antigen is no longer necessary.
The immune response is controlled by several factors:
i. Regulation by antigen.
ii. Regulation by antibody.
iii. Regulation by immune-complexes.
iv. Regulation by lymphocytes.
v. Idiotypic modulation of responses.
vi. Neuroendocrine modulation of immune responses.
vii. Genetic control of immune responses.
viii. MHC-Linked immune response genes.
ix. Non-MHC linked immune response genes.
The main components of the immune system are T-cells and B-cells. In absence of antigen, T and B-cells remain inactive and immune response does not occur. An effective immune response starts when T-cells and B-cells are triggered by antigens.
At the end of effective response, when the antigens are removed or destroyed, then the cells return again to the resting stage. Hence the immune responses is regulated by the antigens.
Antibody itself is capable to exert a form of feedback control on immune response. Passive administration of IgM antibody together with antigen enhances the immune response to the antigen whereas IgG antibody suppresses the response. The mechanism by which antibody regulates the immune response is not completely known.
Immune-complexes can act either to inhibit or augment an immune response. When the B- cell’s Fc receptor is cross-linked to its antigen receptor by an antigen-antibody complex, a signal is delivered to the B-cell inhibiting it from entering the antibody production phase. Antibody encourages presentation of antigen to B-cells when it is present on an antigen presenting cell (APC) bound via Fc receptors.
T lymphocytes regulate the immune response. There is also a clear evidence that T-lymphocytes are capable of down-regulating immune response. T-lymphocytes in immune system stimulates either humoral or cell- mediated immunity.
It has been observed that CD| T-cells are generated following administration of high doses of auto-antigen and prevent further induction of autoimmunity. The exact mechanism by which T-lymphocytes exert such a negative response is not entirely clear. Recently, it has been suggested that the production of cytokines by T-lymphocytes can either partially or totally suppress an immune response.
Idiotype means the antigenic characteristic of the antigen binding site of an antibody. Antibodies formed against these antigen binding sites are called anti-idiotypic antibodies and they are capable of influencing the outcome of an immune response.
When an antibody response is triggered by antigen this antibody will, in turn, invoke an anti-idiotypic response to itself. There is good evidence that anti-idiotypes can affect the representation of recognised idiotypes in an immune response.
Immune response is also regulated by the influences of the nervous and endocrine systems. It is well-known that stressful condition may suppress the immune response. The nervous system directly or indirectly controls the output of various hormones which, in turn, also modulate immune function.
Gene controls everything at the cellular level. Immune response is also under the genetic control. There are many ways in which Genetic factors may play some role in influencing the immune response. Pedigree analysis and/or recording a family history of susceptibility to any infection will suggest that resistance or susceptibility may be an inherited characteristic.
Again, variation among individuals in respect to immune response is mainly due to combination of different gene(s) or due to lack of combination of gene(s) controlling the production of mediators of immunity.
The immune response is also controlled by MHC-linked immune response (Ir) genes. It is well-established that the immune response depends upon the activation of lymphocytes. The activation of T-lymphocyte is induced by MHC molecules.
It is now established that during development, T-lymphocytes are subjected to two selection processes in the thymus:
i. Positive selection, based on an interaction of TCR with MHC on thymic cortical epithelium.
ii. Negative selection as result of a high affinity interaction between TCR and MHC peptide presented on bone marrow derived cells in the thymus medulla.
The extensive sequence polymorphism of MHC molecules governed by Ir genes has a profound impact on T-cell activation. Thus both positive and negative selection of T-cell affect the immune response.
MHC-linked genes have been shown to play a role in the immune response to infectious agents and also to self-antigens. In some cases, the gene involved is an MHC gene itself, but in others it is thought to be a gene that is simply linked to the MHC. MHC-linked gene have been found to influence autoimmune diseases.
The immune response is modulated by some genes outside the MHC region. Macrophages play a key role in the immune system. Genes regulating their activity may, therefore, determine the outcome of many immune responses. Most autoimmune diseases have been shown to be influenced not only by MHC-linked genes, but also by genes not linked to this complex.
5. Immunoassay Techniques:
Many of the in vivo antigen-antibody reactions can also be carried out in controlled laboratory conditions, i.e., under in vitro conditions and these are extensively used in diagnostic testing. This branch of immunology concerned with in vitro antigen-antibody reactions is termed serology (study of serum).
A few of these reactions have been taken up for consideration:
(i) Agglutination Tests:
Immune complex is formed by the cross-linking of cells or particles with specific antibodies. This is termed agglutination. Agglutination reactions usually form visible aggregates or clumps that may be observed with the unaided eye.
Direct agglutination reactions are useful in diagnosis of certain diseases. Thus, Widal test is reaction involving agglutination of typhoid bacilli when they are mixed with serum containing typhoid antibody from an individual who has typhoid.
Agglutination tests may be used to measure antibody concentration. In a tube or well agglutination test, a specific amount of antigen is added to series of tubes (Fig. 21.44) or shallow wells in what is known as microtiter plate.
Serial dilutions of serum 1/20, 1/40, 1/80, 1/160 etc. Antibody are then added to each tube (Fig. 21.45) or well. The greatest dilution of serum, showing an agglutination reactions is determined. The reciprocal of this dilution is the serum antibody concentration.
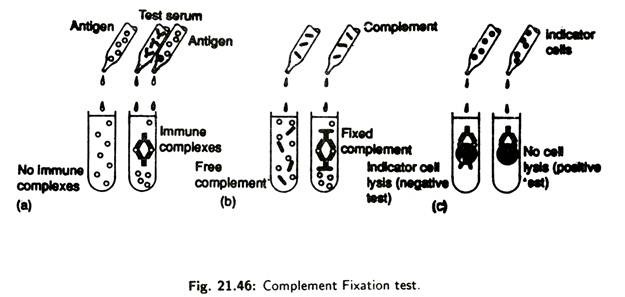
(ii) Component Fixation Tests:
When complements bind to an antigen- antibody complex it becomes fixed or used up. Complement fixation tests (Fig. 21.46) are very sensitive and can be used to detect extremely small amounts of antibody for a suspected microorganism in an individual’s serum.
A known antigen is mixed with test serum lacking complement. After allowing immune complex to form, the complement is added to the mixture. If immune complexes are present they will fix and consume the complement.
Next, sensitized indicator cells, usually sheep red blood cell, previously coated with complement fixing antibody, are added to the mixture. Lysis of the indicator cells results, if immune complexes do not get formed in the initial part of the test.
This is because antibodies are not present in the test serum, and, in the absence of the antibodies, complement remains and causes lysis of the indicator cells. However, if specific antibody are present in the test serum and complement is consumed by the immune complexes, insufficient amount of complement will be available to lyse the indicator cells.
Absence of lysis shows that specific antibodies are present in the test serum. Complement fixation tests are used in diagnosis of certain viral and protozoan diseases.
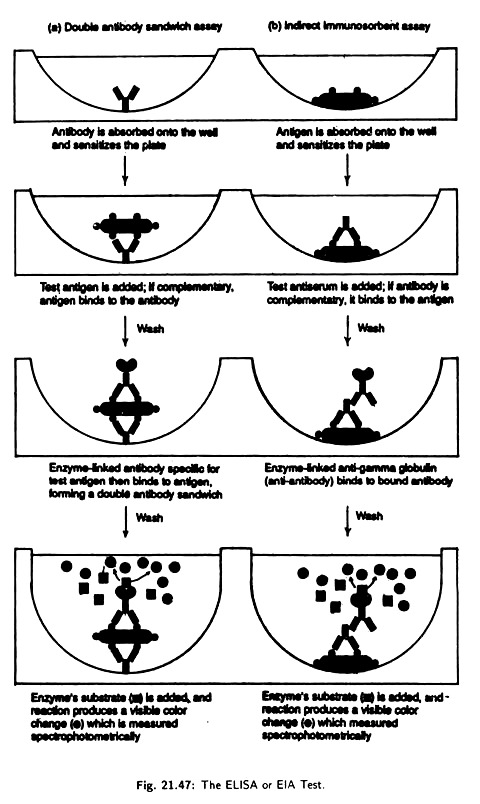
(iii) Enzyme Linked Immunosorbent Assay (ELISA):
This has become one of the most widely used serological tests for antibody or antigen detection. This test involves linking of various label enzymes to either antigens or antibodies.
There are two basic methods:
i. The Double Antibody Sandwich Assay; and
ii. The Indirect Immunosorbent Assay.
i. Double antibody sandwich assay:
It is used for the detection of antigens. In this assay specific antibody is placed in the well of a microtiter plate [Fig. 21.47(a)]. The antibody gets absorbed into the walls sensitizing the plate. A test antigen is then added to each well.
If the antigen reacts with the antibody, the antigen is retained when the well is worked to remove unbound antigen. Next an antibody- enzyme conjugate specific for the antigen is added to each well. The final complex is formed of an outer antibody-enzyme complex, middle antigen and inner antibody layer, i.e., it is a layered antibody-antigen-antibody sandwich.
A substrate that enzyme will convert to a coloured product is then added and the resulting product is quantitatively measured by optical density scanning of the plate. If the antigen has reacted with the absorbed antibodies in the first step, the Enzyme Linked Immunosorbent Assay test is positive.
If the antigen is not recognised by the absorbed antibody the test is negative, because the unattached antigen has been washed away and no antibody-enzyme is bounded. This assay is used for detection of causal agents of syphilis, salmonellosis etc.
ii. Indirect immunosorbent assay:
This detects antibodies rather than antigens. Antigens in appropriate sensitizing buffer axe incubated in the wells of a microtiter plate [Fig. 21.47(b)] and is absorbed on the walls of the well. Free antigen is then washed away. Alternatively the test sample can be incubated with a suspension of latex bars that have the desired antigen attached to their surface.
After allowing antibody-antigen complex to form the breeds conjugate are trapped on a filter and unbound antibody is washed away. An antibody covalently linked to an enzyme (Antibody-enzyme conjugate) is then added.
The antibody-antigen complex then bind to the test antibody. Any unbound antibody- enzyme conjugate is washed away. The combined complex is next visualised by addition of a chromogen.
A chromogen is a colourless substrate acted on by the enzyme portion of the complex to produce a coloured product the amount of test Antibody is next accessed in the same way was the antigen is accessed in the double antibody sandwich method. The test is used to test Antibody for AIDS, German Measles and to detect certain drugs in urine (Dope Test for athletes).
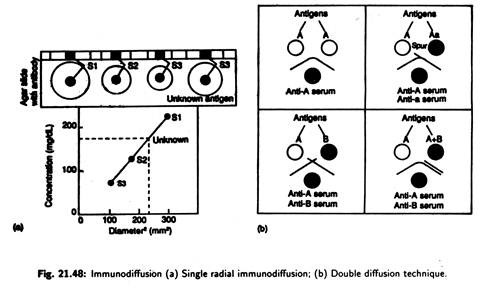
(iv) Immunodiffusion:
It refers to a precipitation reaction that occurs between an antibody and an antigen on an agar gel medium.
Two procedures are in practice:
Single radial immunodiffusion assay or mancini’s technique:
Mono-specific antibody is added to agar and mixture is then poured into slides and allowed to set; wells are cut into the agar, and known amount of standard antigen is added [Fig. 21.48(a)]. This unknown test antigen is added to a separate well.
The slide is left for 24 hours. The antigen diffuses out of the well to forms an insoluble complex. The size of the resulting precipitation ring surrounding various dilutions of antigen selected is proportional to the amount of Antigen in the well.
The wider the ring the greater is the concentration. The antigen form a precipitin ring in the agar when its level has decreased sufficiently to reach the optimal level when it combines with the antibody to produce large insoluble network.
Double diffusion technique or ouchterlony’s technique:
Here double diffusion, i.e., diffusion of both antibody and antigen through agar, takes place to produce easily observable immune complexes. Test solution of antigen and antibody are added to separate wells punched in agar.
The solution diffuses outward, and, when antigen and appropriate antibody meet, they combine and precipitate at the equivalence zone producing an indicator arc [Fig. 21.48(b)]. Depending upon the line of precipitation (indicator axe), identity of antigens may be established.
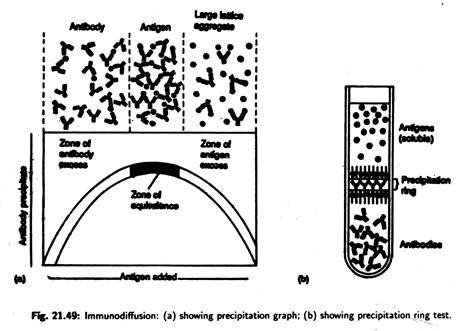
(v) Immunoprecipitation:
Immunoprecipitation is a technique by which soluble antigens can be detected. In the process these soluble antigens react with antibodies called precipitins. A precipitin reaction occurs when bivalent or multivalent antigens and antibodies are mixed in the proper proportion.
Antibodies link to the antigens to form a large antibody-antigen network or lattice that settles out of solution when it becomes sufficiently large [Fig. 21.49(a)]. Immunoprecipitation reactions occur only at the equivalence zone, where there is an optimal ratio of the antigen to antibody so that the lattice forms.
If the precipitin reaction is carried out in a test-tube [Fig. 21.49(b)], a precipitation ring forms in the area in which the optimal ratio or equivalence zone develops.
(vi) Immunoelectrophoresis:
Some antigen mixtures are too complex to be resolved by simple diffusion and precipitation. Greater resolution may be obtained by the technique of immuno-electrophoresis in which antigen are first separated based on their electric charge (Fig. 21.50), then visualised by the precipitation reaction.
In the procedure, antigens are separated by the electrophoresis in an agar gel—positively charged proteins move towards the negative electrode and negatively charged proteins move towards the positive electrode, although there is a cut near the antigta wells and filled with antibody.
The plate is then incubated, the antigens and antibodies diffuse, and, eventually, form precipitation bands, which can be better visualised by staining.
Immunoelectrophoresis is used to separate major blood proteins in serum for certain diagnostic tests.
(vii) Immunofluorescence:
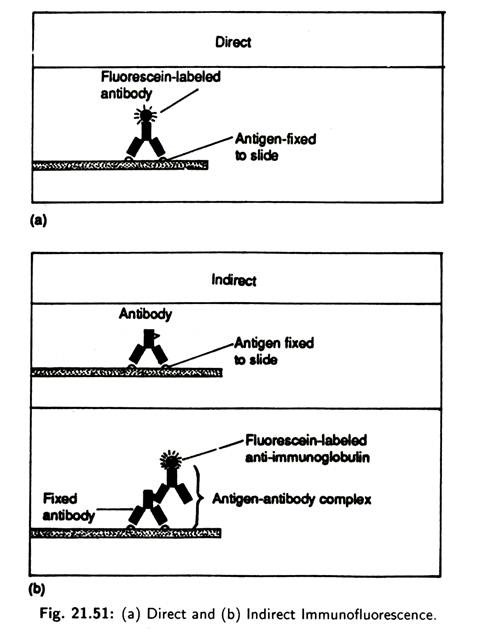
Immunofluorescence is the process in which dyes called fluorochromes are exposed to ultraviolet, violet or blue light to make them fluoresce or emit visible light. Dyes like rhodamine B or fluorescein isothiocyanate can be utilised to antibody’s capacity to bind to a specific antigen. Fluorochromes also can be attached to antigens. Fluorescent antibody assays may be direct or indirect (Fig. 21.51).
Direct immunofluorescence involves fixing the specimen (cell or microorganisms) containing the antigen of interest into a slide. Fluorescence-labelled antibodies are then added to the slide and incubated. The slide is washed to remove any unbound antibody and examined with a fluorescent microscope for a yellow-green fluorescence.
The pattern of fluorescence reveals the antigen location. Direct immunofluorescence is used to identify antigens found on surface of certain streptococci and other microbes like Salmonella typhi, Hemophilus influenzae, Rabies virus, etc.
Indirect immunofluorescence is used to detect the presence of antibodies in serum following an individual’? exposure to microorganisms. In the process, a known antigen is fixed onto a slide. Test antiserum is added, and, if the antibody is present, it reacts with the antigen to form a complex.
When fluorescein labelled anti-immunoglobulin is added it reacts with the antigen to form a complex. When fluorescein labelled anti-immunoglobulin is added it reacts with the fixed antibody. After incubation and washing, the slide is examined with the fluorescence microscope. If fluorescence is observed then the test antigen is present in the serum.
(viii) Radioimmunoassay:
This is an extremely important biomedical research technique, which also helps in clinical practices such as blood banking, allergy diagnosis, endocrinology etc. These techniques were developed by Rosalyn Yalow.
Radioimmunoassay uses a radio-isotope- labelled purified antigen which competes for binding to an antibody with an un-labelled standard, i.e., antigen in the experimental sample is not known.
Radioactivity associated with the antibody is detected by means of radioisotope analysers. If the concentration of antigen in the experimental sample is high, it competes with the standard antigen for binding sites in the antibody. Consequently, relatively less amount of bound radioactivity indicates that little amount of antigen is present in the experimental sample.
(ix) Hybridomas-Production of Monoclonal Antibodies:
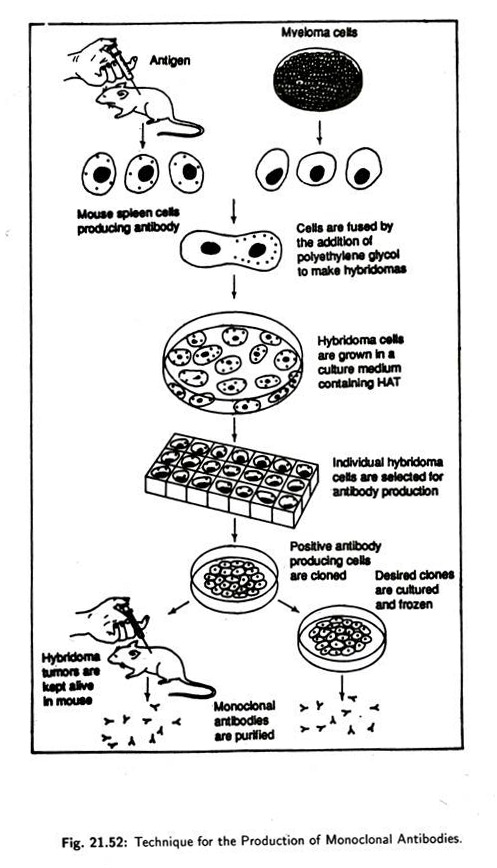
Hybridomas technique for monoclonal antibody production was first developed by Georges Kohler and Cesar Milstein in 1975 in Cambridge, England and for this discovery they (with Niels Jerne) were awarded the Nobel Prize in 1984.
The methodology of producing hybridomas is:
Animals, usually mice or rats are injected with an antigen (Fig. 21.52). The B cells of the mice were thus induced to produce antibodies against that antigen. Once a large number of antibodies start being produced, the spleen of the mice are removed, the spleen cells are separated from each other and are fused with myeloma cells by the addition of polyethylene glycol which promotes membrane fusion.
Myeloma cells are nothing but cancerous cells which are capable of proliferating rapidly under laboratory conditions. Only those myeloma cells are used which are incapable of producing immunoglobulin’s. These fused cells, derived from spleen cells and myeloma cells, are termed hybridomas (i.e., hybrid of two cells).
These hybridomas then grow a culture and, as anticipated they possess some characteristics such as:
i. Capacity of antibody production, imparted by spleen cells.
ii. That of immortality imparted by the cancerous myeloma cells.
The hybridoma cells are individually tested for the production of desired antibody—if the result is positive then that particular type of cells are cloned. The progeny cells produce only that type of antibody which is characteristic to the parent B cell. Thus hybridomas are seen to produce monoclonal antibodies.
(x) Hybridoma Technique:
Production of Monoclonal parent B cell. Thus hybridomas are seen to produce monoclonal antibodies.
Hybridomas found are a permanent union of parent cells. Cells are uniform, highly specific and can be readily produced in large quantities.
Monoclonal antibodies are useful in clinical procedures for the treatment of some diseases. For example is monoclonal antibodies are made for antigens.
Hybridomas formed are apparent union of parent cells. Cells are uniformly specific and can be readily produced in large quantities.
Monoclonal antibodies are useful in clinical procedures for the treatment of some diseases. For example if monoclonal antibodies are made for antigens unique to particular pathogens, these antibodies can be used to treat those particular diseases. Besides monoclonal antibodies are being used to diagnose allergies and infectious diseases like hepatitis, rabies etc.
Early stages of cancer may be detected using monoclonal antibodies because certain types of cancer cells have surface antigens that differ from those of normal cells. Monoclonal antibodies used alone or chemically attached to drugs could locate and destroy cancer cells or pathogenic microorganisms without damaging the surrounding healthy tissues.
6. Inflammation:
Inflammation is an important non-specific defense reaction to tissue injury caused by a pathogen or a wound. Both physiological and biochemical changes are involved for inflammatory response. The physiological changes lead to five classical symptoms: redness, heat, pain, swelling and altered function. The net effect is defensive in nature.
The major physiological events that occur during an inflammatory reaction are:
i. Normally the diameter of the arteriole controls the blood flow into the capillary network. Inflammation causes arteriole dilatation and increased blood flow (hyperemia) into the affected tissue. The capillary becomes distended and produces the gross red-purple discoloration of tissue due to an increased blood content.
ii. The junction between capillary endothelial cells are sufficiently tight to keep the macromolecules within lumen. In inflammation, gaps are formed that allow leakage of fluid and macromolecules into the interstitial space. The fluid leakage produces swelling (edema).
iii. Normally, leucocytes are carried in the lumen of a capillary. During inflammation, the leucocytes adhere to the wall of capillaries, insert pseudopods between endothelial cells and squeeze through by the process of diapedesis.
iv. After leaving the capillaries, the leucocytes are attracted to the source of the inflammation by various chemotactic or chemo- kinetic substances. Once at the infection site, leucocytes engulf (phagocytosis) the microorganism or dead tissue.
The major biochemical events that take place during inflammation are:
i. Cells are ruptured in the infected area and release their cytoplasmic contents.
ii. It raises the acidity in the surrounding extracellular fluid. This decreases the activity of the extracellular enzyme kallikrein. The enzyme splits into bradykinin.
iii. Bradykinin binds to receptors on the capillary wall and opens the junction between cells. This allows the fluid and infection fighting leucocytes to leave the capillary and enter the infected tissue.
iv. At the same time bradykinin also binds to mast cells in the connective tissue associated with most small blood vessels. This activates the mast cells by causing an influx of calcium ions which leads to degranulation and release of preformed mediators such as histamine.
v. If nerves in the infected area are damaged, they release substance P which also binds to mast cells and promotes the release of histamine. It makes the capillary wall wider so that more fluid, leucocyte, kallikrein and bradykinin move out and cause edema.
vi. Bradykinin binds to nearby capillary cells and stimulates the production of prostaglandins to promote tissue swelling in the infected area. Prostaglandins also bind to free nerve endings and start a pain impulse.
vii. The change in mast cell plasma membrane permeability is involved with activation of the enzyme phospholipase A2 and allows it to release arachidonic acid. This acid then follows either cyclooxygenase or lipoxygenase pathways depending on mast cell type and produces newly synthesised mediators that lead to the inflammatory response.
7. Immunisation:
Immunization means the artificial induction of immunity to disease. The procedure of immunization is very simple. In most cases, mild doses of purified antigen of a particular disease is introduced into the body of a healthy person.
The host’s immune system recognises and responds to the antigen and its B cells proliferate and differentiate to produce specific antibody, naturally. As a result, a person becomes resistant to particular pathogen if it attacks naturally. To promote the efficiency of antigen stimulation of antibody production, the antigen is mixed with an adjuvant which enhances the rate and quantity of antibody produced.
Antigenic material that produces specific immunity by stimulating an immune response is termed a vaccine.
Vaccines are of three type:
(a) Killed pathogen;
(b) Attenuated strains (avirulent) of the same pathogen species and
(c) Chemically modified toxins, i.e., toxoids.
The most widely used vaccine of the first type is pertussine. It is a vaccine used against the childhood disease whooping cough which is caused by Bordetella pertussis (bacteria). The first successful vaccine against polio was also a formalin-killed polio virus, but it has been replaced by the Sabin oral polio vaccine which is an attenuated strain of polio virus.
Strains of pathogens that have lost virulence are termed attenuated strains. Except a naturally occurring attenuated strains of small pox virus, attenuated strains of other pathogen are developed in the laboratory.
Most vaccines that are used against viral diseases are strains that have been attenuated in the laboratory. Vaccines against measles, mumps, polio and rubella are examples of live attenuated vaccines. Apparently mutations that occur during the time of attenuation are responsible for the loss of virulence.
Some bacteria like those of tetanus and diphtheria secrete toxin that causes the diseases. Active toxin isolated from such bacteria cannot be used to immunize against these diseases because the amount needed to stimulate an immune response is lethal. However, these toxins can be chemically modified to nontoxic forms—termed toxoids—that retain the ability to stimulate production of toxin-neutralizing antibodies.
Resistance to many diseases can be produced by injecting into a person a preparation containing antibodies that bind specifically to antigens produced by a pathogen. Such resistance is called passive immunity. It is highly effective for several hours after the injection. But vaccines require at least several days to produce resistance. The duration of passive immunity is relatively brief.
Previously antibodies were prepared in a domestic animal by repeated antigen injection at regular intervals. Following antigen injection, blood is withdrawn from the animal’s body and allowed to clot. The fluid that remains after the blood clot is the serum.
Because this serum has been obtained from an immunized animal and contains the desired antibodies, it is called antiserum. Currently, most preparations used for passive immunization contain antibodies termed human immune globulins that are purified either from the serum of a person who has a high degree of immunity to a particular disease or, in the case of disease to which immunity is common, from a collection of serum samples taken from many persons.
Usually a person who has never been exposed to an antigen lacks detectable amount of the corresponding antibody. Then, following exposure to immunization procedure, a primary response occurs: the level of antibody usually remains undetectable for several days and then it rises over a period of one or more weeks, reaching a peak, and, finally, gradually decreasing.
When the person is exposed again to the same immunization procedure, a response called a secondary or anamnestic response follows. In most cases of secondary response, the level of antibody begins to rise much sooner than it did before and it reaches a much higher value.
The immunological basis of the secondary response is the proliferation of TH and B- cells that occur during the primary response. These cells have a long life-span and behave as memory cells in the secondary response. Memory cells are responsible in many cases for acquired immunity.
8. Transplantation Rejection —An Immune Response:
It is occasionally necessary to replace a nonfunctional or damaged body part by transplanting a tissue or organ from one person to another. Such transplantation reaction are of various types. Some transplants do not stimulate an immune response.
Fbr example, a transplanted cornea of eye is not rejected immunologically since lymphocytes do not circulate into the anterior chamber of the eye. In this case there is no question of graft or tissue rejection. Hence this site is considered as an immunologically privileged site.
Transplantation of skin or organs from somebody to another is also popularly known as grafting. It is of different types. When a heart valve is transplanted from a pig to human, such a graft between different species is called a xenograft.
When one’s own tissue is grafted from one part of the body to another (for example, skin from the thigh is transplanted over a burnt area of hand) the graft is not rejected. This type of graft is called an auto-graft.
When a tissue is grafted between two genetically identical individuals such as identical twins, the graft does not initiate any immune response. This type of graft is called isograft.
Usually, however, transplants are done between genetically different individuals within a species. This is termed allografts. Here rejection may occur. With allograft there is the possibility that the recipient’s cell will recognize the donor’s tissue as foreign. Hence it initiates the recipient’s immune mechanism which may destroy tissue. Such a response is termed as graft rejection.
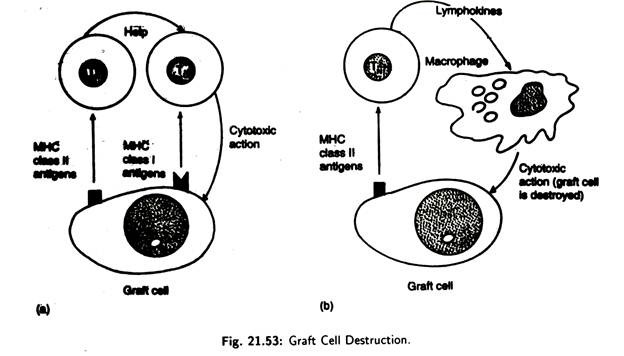
A graft rejection may occur by two different mechanisms:
i. Foreign major histocompatibility complex class II antigens on the graft stimulate host T-helper lymphocytes to aid cytotoxic T-cells in graft destruction [Fig. 21.53(a)]. Cytotoxic T-cells recognize the graft through the foreign MHC class I antigen.
ii. T-helper lymphocytes reacts with the graft and release lymphokines [Fig. 21.53(b)] that stimulate macrophages to enter the graft and destroy it via cytotoxic action.
Prevention of graft rejection can be achieved by using some immunosuppressive drugs such as azathioprine, glucocorticoid steroids, cyclosporin-A, cyclophosphamide and anti-lymphocyte and irradiation.
These measures reduce the possibility of rejection of transplanted tissue as well as interfere with the recipient’s immune mechanism in various ways. They may stop temporarily the formation of antibody by B-cells or the production of T-cells for certain period. During this period immunosuppression measures leave the patient relatively unprotected against infection and cancer.