ADVERTISEMENTS:
This article throws light upon the nine things you must know about cell culture.
The nine things are: (1) What is Cell and Tissue Culture ? (2) Equipment for Cell Culture (3) Subculturing of Cells (4) Cell Quantification (5) Animal Cell Culture (6) How are Cell Cultures Obtained for Culture? (7) Characteristics of Cultured Cells (8) Problems Faced by Cultured Cells and (9) How to Decide if Cultured Cells are “Happy”.
Thing # 1. What is Cell and Tissue Culture ?
Tissue Culture is the general term for the removal of cells, tissues, or organs from an animal or plant and their subsequent placement into an artificial environment conducive to growth.
ADVERTISEMENTS:
This environment usually consists of a suitable glass or plastic culture vessel containing a liquid or semisolid medium that supplies the nutrients essential for survival and growth.
The culture of whole organs or intact organ fragments with the intent of studying their continued function or development is called Organ Culture. When the cells are removed from the organ fragments prior to, or during cultivation, thus disrupting their normal relationships with neighboring cells, it is called Cell Culture. Generally three types of culture are being done. They are animal cell culture, plant cell culture and microbial culture.
Thing # 2. Equipment for Cell Culture:
These include a tissue culture hood, incubators, autoclave and microscopes.
1. Cell Culture Hoods:
This is the central piece as it is the place where all the cell handing is carried out. These hoods are designed not only to protect the culture from operator but also to protect operator from the cultures. They are generally referred to as laminar flow hoods as they generate a smooth, uninterrupted streamlined flow (laminar flow) of sterile air which has been filtered through a high efficiency particulate air (HEPA) filter. There are two types of laminar flow hoods classified as vertical or horizontal.
ADVERTISEMENTS:
The horizontal hood allows the air to flow directly towards operator and thus is generally used for non-infectious work like media preparation, etc. The vertical hoods also called as biological safety cabinets are best for working with hazardous organisms, since air within the hood is filtered before it passes in the surrounding environment.
Currently, there are three different classes of hoods used, which provide various levels of protection to culture, to operator or to both.
Class-I hoods:
These hoods along with class-II have screen at the front which acts as barrier between culture and operator but yet allows access to culture through a opening from bottom of the screen. This provides too much turbulence to air from outside and also provides good protection for the operator. These hoods are suitable for use with low risk organisms and when operator protection only is required.
Class-II hoods:
These are most commonly used hoods. Unlike class-I, air drawn from outside is passed through the grill in front of the work area and filtered through the HEPA filter at the top of the hood before streaming down over the tissue culture. This mechanism not only protects the operator but also ensures the air over the culture is largely sterile. The hoods are adequate for animal cell culture, which involves low to moderate toxic or infectious agents, but are not recommended for use with high risk pathogens.
Class-Ill hoods:
These are required when highest level of operator and product protection is required. These hoods are completely sealed, providing two gloves pockets through which operator can work with material inside the cabinet. Thus the operator is completely shielded making class-Ill hoods suitable to use with high risk involving cultures.
ADVERTISEMENTS:
Safety aspects:
All hoods must maintain culture-free and clean state at all times as too much culture may affect air flow, and contamination may introduce infections. Thus only those dedicated items must be placed inside the hoods that are required, and clean all the work places before and after use with 70% industrial methylated sprit (IMS) which acts against bacterial and fungal spores by dehydrating and fixing cells, thus preventing contamination of cultures.
Some cabinets may be equipped with a short wave ultraviolet light that can be used to irradiate the interior of the hood and to kill micro-organisms. When present, switch on the UV light 15 min before work to sterilize the work place. As UV can cause adverse effect to skin and eyes, precautions must be taken at all times that operator is not in direct contact with UV. Additionally always turn on the hood at least 10 min prior to start of work to allow the flow of air to stabilize.
2. CO2 Incubators:
Water jacketed incubators are required to facilitate optimum cell growth under strictly maintained and regulated conditions, normally requiring a constant temperature of 37°C and an atmosphere of 5-10% CO2 plus air. The purpose of CO2 is to ensure that culture medium is maintained at required physiological pH (usually 7.2-7.4).
ADVERTISEMENTS:
This is achieved by the supply of CO2 by glass cylinder into the incubator through a wall that is triggered to draw in CO2 whenever the level falls below the set level. The CO2 that enters dissolves in the culture medium contain bicarbonate.
The latter reacts with H+ generated from cellular metabolism to form carbonic acid, which is in equilibrium with water and CO2, thereby maintaining the pH in the medium at approximately 7.2. These incubators are generally humidified by a tray of sterile water at the bottom deck. The evaporation of water creates highly humidified atmosphere which helps to prevent evaporation of medium from culture.
An alternative to humidified incubators is the dry non-gassed unit that is not humidified and relies on the use of alternative buffering systems such as 4(2-hydroxyethyll)-l-piperazine-ethanesulphonic acid (Hepes) or morpholinopropane sulphonic acid (mops) for maintaining a balanced pH within the culture medium. The advantage of this is that it can rule out the chances of infection by water tray. The disadvantage, however, is that culture medium will evaporate fast thereby stressing the cells.
3. Microscopes:
Inverted phase contrast microscopes are usually employed for visualizing cells in culture medium. They are expensive but easy to operate with light source located above and the objective lenses below the stage on which the cells are placed. Visualization of cells by microscopy provides useful information about morphology and state of the cells. Early signs of stress can easily be identified and thus precautionary measures can be taken.
4. Other General Equipment’s:
These include centrifuges to spin down the cells, water baths for thawing frozen samples and warming media to 37°C before starting the work, and refrigerators for storing media, etc. Some cells need to attach on a surface to grow, for this purpose non-toxic polystyrene plastics containing biologically inert surface on which cells can grow are available. Various kinds of plastics are available for this purpose which include Petri dishes, multi-well plates, and screw cap flasks.
Thing # 3. Subculturing of Cells:
It is a process by which cells are harvested, diluted in fresh growth medium and replaced in a new culture flask to promote further growth. This process is known as passaging, which is essential if the cells are to be maintained in a healthy and viable state, otherwise they may die after a certain period in continuous culture. The reason for this is that adherent cells grow in continuous layer that eventually occupy the whole surface of the culture dish and at this point they are said to be confluent.
ADVERTISEMENTS:
Once confluent, the cells stop dividing and go into a resting state where they stop growing and eventually die. Thus to keep cells viable and to facilitate efficient transformation, they must be sub-cultured before they reach full contact inhibition. Ideally, cells should be harvested just before they reach confluent state. Cells can be harvested and sub-cultured using one of several techniques. The precise method used is dependent to a large extent on whether the cells are adherent in suspension.
Subculture of Adherent Cells:
Adherent cells can be harvested either mechanically, usually a rubber spatula (rubber policeman) or enzymatically using proteolytic enzymes. Cells in suspension are simply diluted in fresh medium by taking a given volume of cell suspension and adding an equal volume of medium.
Harvesting of Cells Mechanically:
This method is simple and easy. This involves gentle scrapping of cell from growth surface into the culture medium using a rubber spatula. This method is not suitable for all cell types as the scrapping may result in membrane damage and significant cell death.
Harvesting of Cells Using Proteolytic Enzymes:
Several different proteolytic enzymes can be exploited including trypsin, proteolytic enzymes that destroys proteinaceous connections between cells and the surface of the flask in which they grow. As a result harvesting of cells using this enzyme results in the release of single cells, which is ideal for sub-culturing as each cell will then divide and grow, thus enhancing the propagation of cultures.
Trypsin is commonly used with EDTA, which enhances the action of enzyme. EDTA alone can also be effective in detaching adherent cells as it chelates the Ca2+ required by some adhesion molecules that facilitate cell-cell or cell-matrix interactions. Although EDTA alone is much gender on the cells than trypsin, some cell types may adhere strongly to plastic, requiring trypsin to detach.
ADVERTISEMENTS:
The standard procedure for detaching adherent cells using trypsin and EDTA involves making a working solution of 0.1% trypsin plus 0.02% EDTA in Ca2+/Mg2+ free phosphate-buffered saline. The growth medium is aspirated from confluent cultures and washed at least twice with a serum-free medium such as Ca2+/Mg2+ free PBS to remove traces of serum that may inactivate the trypsin.
The trypsin-EDTA is then added to cell monolayer and swirled around for a few seconds. Excess trypsin-EDTA is aspirated, leaving just enough to form a thin film over the monolayer. The flask is then incubated at 37°C for 2-5 min but monitored under an inverted light microscope when cells are beginning to round up and detach. This is to ensure that cells are not overexposed to trypsin and cell surface may get damaged.
It is, therefore, important that proteolysis reaction is appropriately terminated by adding complete medium having serum to deactivate the enzyme. The suspension of cells is then subjected to centrifuge and the pellet is re-suspended in known volume of complete medium to give a required density of cells. All of the above procedure is carried out in a sterile tissue culture cabinet.
Subculture of Cells in Suspension:
For cells in suspension it is important initially to examine an aliquot of cells under a microscope to establish whether cultures are growing as single cells or clumps. If cultures are growing as a single cell, an aliquot is counted and then reseeded at desired seeding density in a new flask by simply diluting the cell suspension with fresh medium, provided the original medium is not spent. Cells that grow in clumps should first be centrifuged and re-suspended in fresh medium as single cell using a glass Pasteur or fine-bore pipette.
Thing # 4. Cell Quantification:
It is essential that when cells are sub-cultured they are reseeded in appropriate density so as to facilitate optimum growth. If cells are seeded at lower seeding density they may take longer time to reach confluency and some may expire before getting this point.
On the other hand, if seeded in high density they will reach confluency very quickly, resulting in irreproducible experimental results. This is because trypsin may digest surface proteins, including receptors for drugs and these may take time to renew. Therefore, appropriate seeding density is a must which requires quantification of the cells.
It involves the use of haemocytometer, which is a thickened glass slide with small chambers of grids. The chamber has a fixed volume and is etched into nine large squares, of which the large corner square contain 16 small square each; each large square measures 1 mm × 1mm and is 0.1 mm deep. Thus with a coverslip in place, each square represents a volume of 0.1 mm3. Knowing this, cell concentration can be determined and expressed per cubic centimeters.
Another alternative method available for cell quantification is use of electronic coulter counter. This is an automated method of counting and measuring size of microscopic particles. The instrument consists of glass probe with an electrode connected to an oscilloscope. The probe has a small aperture of fixed diameter near its bottom end.
When immersed in solution of cell suspension, cells are flushed through the aperture causing the brief increase in resistance owing to a partial interruption of current flow. This will result in spikes being recorded on oscilloscope and each spike is counted as a cell. The disadvantage this technique had is, it cannot distinguish between live and dead cells. Also indirectly cells can be counted by determining total cell protein and using protein versus cell number standard curve to determine cell number in test samples.
Again, the DNA content of cells may be used as an indicator of cell number, since the DNA content of diploid cells is usually constant. However, the DNA content of cells may change during the cell cycle and, therefore, does not give an accurate estimate of cell number.
Thing # 5. Animal Cell Culture:
Although animal cell culture was first successfully undertaken by Ross Harrison in 1907, it was not until the late 1940s to early 1950s that several developments occurred that made cell culture widely available as a tool for scientists.
First, there was the development of antibiotics that made it easier to avoid many of the contamination problems that plagued earlier cell culture attempts.
Second was the development of the techniques, such as the use of trypsin to remove cells from culture vessels, necessary to obtain continuously growing cell lines (such as HeLa cells).
Third, using these cell lines, scientists were able to develop standardized, chemically defined culture media that made it far easier to grow cells.
These three areas combined to allow many more scientists to use cell, tissue and organ culture in their research. During the 1960s and 1970s, commercialization of this technology had further impact on cell culture that continues to this day.
Companies, such as Corning, began to develop and sell disposable plastic and glass cell culture products and improve filtration products and materials, liquid and powdered tissue culture media, and laminar flow hoods. The overall result of these and other continuing technological developments has been a widespread increase in the number of laboratories and industries using cell culture today.
Thing # 6. How are Cell Cultures Obtained for Culture?
Primary Culture:
When cells are surgically removed from an organism and placed into a suitable culture environment, they will attach, divide and grow. This is called a Primary Culture. There are two basic methods for doing this.
First, for Explant Cultures, small pieces of tissue are attached to a glass or treated in plastic culture vessel and bathed in culture medium. After a few days, individual cells will move from the tissue explant onto the culture vessel surface or substrate where they will begin to divide and grow.
The second, more widely used method speeds up this process by adding digesting (proteolytic) enzymes, such as trypsin or collagenase, to the tissue fragments to dissolve the cement holding the cells together. This creates a suspension of single cells that are then placed into culture vessels containing culture medium and allows to grow and divide. This method is called Enzymatic Dissociation.
Sub-culturing:
When the cells in the primary culture vessel have grown and filled up all of the available culture substrate, they must be sub-cultured to give them room for continued growth. This is usually done by removing them as gently as possible from the substrate with enzymes. These are similar to the enzymes used in obtaining the primary culture and are used to break the protein bonds attaching the cells to the substrate.
Some cell lines can be harvested by gently scraping the cells off the bottom of the culture vessel. Once released, the cell suspension can then be subdivided and placed into new culture vessels. Once a surplus of cells is available, they can be treated with suitable cryoprotective agents, such as dimethylsulphoxide (DMSO) or glycerol, carefully frozen and then stored at cryogenic temperatures (below — 130°C) until they are needed.
Buying and Borrowing:
An alternative to establishing cultures by primary culture is to buy established cell cultures from organizations such as the American Type Culture Collection or the Coriell Institute for Medical Research. These two nonprofit organizations provide high quality cell lines that are carefully tested to ensure the authenticity of the cells. More frequently, researchers will obtain (borrow) cell lines from other laboratories.
While this practice is widespread, it has one major drawback. There is a high probability that the cells obtained in this manner will not be healthy, useful cultures. This is usually due to previous mix-ups or contamination with other cell lines, or the result of contamination with micro-organisms such as mycoplasmas, bacteria, fungi or yeast.
Thing # 7. Characteristics of Cultured Cells:
Once in culture, cells exhibit a wide range of behaviours, characteristics and shapes.
Some of the more common ones are described below:
Cell Culture Systems:
Two basic culture systems are used for growing cells. These are based primarily upon the ability of the cells to either grow attached to a glass or treated plastic substrate (Monolayer Culture Systems) or floating free in the culture medium (Suspension Culture Systems).
Monolayer cultures are usually grown in tissue culture treated dishes, T-flasks, roller bottles, or multiple well plates, the choice being based on the number of cells needed, the nature of the culture environment, cost and personal preference.
Suspension cultures are usually grown either:
1. In magnetically rotated spinner flasks or shaken Erlenmeyer flasks where the cells are kept actively suspended in the medium.
2. In stationary culture vessels such as T-flasks and bottles where, although the cells are not kept agitated, they are unable to attach firmly to the substrate. Many cell lines, especially those derived from normal tissues, are considered to be Anchorage-Dependent, that is, they can only grow when attached to a suitable substrate.
Some cell lines that are no longer considered normal (frequently designated as Transformed Cells) are frequently able to grow either attached to a substrate or floating free in suspension; they are Anchorage-Independent. In addition, some normal cells, such as those found in the blood, do not normally attach to substrates and always grow in suspension.
Types of Cells:
Cultured cells are usually described based on their morphology (shape and appearance) or their functional characteristics.
There are three basic morphologies:
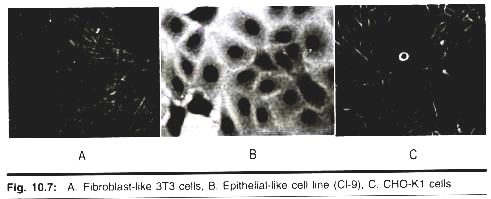
1. Epithelial-like:
Cells that are attached to a substrate and appear flattened and polygonal in shape.
2. Lymphoblast-like:
Cells that do not attach normally to a substrate but remain in suspension with a spherical shape.
3. Fibroblast-like:
Cells that are attached to a substrate and appear elongated and bipolar, frequently forming swirls in heavy cultures. It is important to remember that the culture conditions play an important role in determining shape and that many cell cultures are capable of exhibiting multiple morphologies.
Using cell fusion techniques, it is also possible to obtain hybrid cells by fusing cells from two different parents. These may exhibit characteristics of either parent or both parents. This technique was used in 1975 to create cells capable of producing custom tailored monoclonal antibodies. These hybrid cells (called Hybridomas) are formed by fusing two different but related cells. The first is a spleen-derived lymphocyte that is capable of producing the desired antibody.
The second is rapidly dividing myeloma cell machinery for making antibodies but is not programmed to produce any antibody. The resulting hybridomas can produce large quantities of the desired antibody. These antibodies, called Monoclonal Antibodies due to their purity, have many important clinical, diagnostic, and industrial applications with a yearly value of well over a billion dollars.
Functional Characteristics:
The characteristics of cultured cells result from both their origin (liver, heart, etc.) and how well they adapt to the culture conditions. Biochemical markers can be used to determine if cells are still carrying on specialized functions that they performed in vivo (e.g., liver cells secreting albumin). Morphological or ultra structural markers can also be examined (e.g., beating heart cells).
Frequently, these characteristics are either lost or changed as a result of being placed in an artificial environment. Some cell lines will eventually stop dividing and show signs of aging. These lines are called Finite. Other lines are, or become immortal; these can continue to divide indefinitely and are called Continuous cell lines. When a “normal” finite cell line becomes immortal, it has undergone a fundamental irreversible change or “transformation”. This can occur spontaneously or be brought about intentionally using drugs, radiation or viruses.
Transformed Cells are usually easier and faster growing, may often have extra or abnormal chromosomes and frequently can be grown in suspension. Cells that have the normal number of chromosomes are called Diploid cells; those that have other than the normal number are Aneuploid. If the cells form tumours when they are injected into animals, they are considered to be neo-plastically transformed.
Thing # 8. Problems Faced by Cultured Cells:
Avoiding Contamination:
Cell culture contamination is of two main types: chemical and biological. Chemical contamination is the most difficult to detect since it is caused by agents, such as endotoxins, plasticizers, metal ions or traces of chemical disinfectants, that are invisible.
Biological contaminants in the form of fast growing yeast, bacteria and fungi usually have visible effects on the culture (changes in medium turbidity or pH) and thus are easier to detect (especially if antibiotics are omitted from the culture medium). However, two other forms of biological contamination, mycoplasmas and viruses, are not easy to detect visually and usually require special detection methods.
There are two major requirements to avoiding contamination. First, proper training in the use of good aseptic technique on the part of the cell culturist. Second, properly designed, maintained and sterilized equipment, plastic ware, glassware, and media. The careful and selective (limited) use of antibiotics designed for use in tissue culture can also help avoid culture loss due to biological contamination.
Finding a “Happy” Environment:
To cell culturists, a “happy” environment is one that does more than just allow cells to survive in culture. Usually, it means an environment that, at the very least, allows cells to increase in number by undergoing cell division (mitosis). Even better, when conditions are just right, some cultured cells will express their “happiness” with their environment by carrying out important in vivo physiological or biochemical functions, such as muscle contraction or the secretion of hormones and enzymes. To provide this environment, it is important to provide the cells with the appropriate temperature, a good substrate for attachment, and the proper culture medium.
Temperature is usually set at the same point as the body temperature of the host from which the cells were obtained. With cold-blooded vertebrates, a temperature range of 18-25°C is suitable; most mammalian cells require 36-37°C. This temperature range is usually maintained by use of carefully calibrated, and frequently checked, incubators. Anchorage-dependent cells also require a good substrate for attachment and growth.
Glass and specially treated plastics (to make the normally hydrophobic plastic surface hydrophilic or wettable) are the most commonly used substrates. However, attachment factors, such as collagen, gelatin, fibronectin and laminin, can be used as substrate coatings to improve growth and function of normal cells derived from brain, blood vessels, kidney, liver, skin, etc. Often normal anchorage-dependent cells will also function better if they are grown on a permeable or porous surface.
This allows them to polarize (have a top and bottom through which things can enter and leave the cell) as they do in the body. Trans well inserts are corning vessels with membrane-based permeable supports that allow these cells to develop polarity and acquire the ability to exhibit special functions such as transport. Many specialized cells can only be truly “happy” (function normally) when grown on a porous substrate in serum-free medium with the appropriate mixture of growth and attachment factors.
Cells can also be grown in suspension on beads made from glass, plastic, polyacrylamide and cross-linked dextran molecules. This technique has been used to enable anchorage-dependent cells to be grown in suspension culture systems and is increasingly important for the manufacture of cell- based biological. The culture medium is the most important and complex factor to control in making cells “happy”.
Besides meeting the basic nutritional requirement of the cells, the culture medium should also have any necessary growth factors, regulate the pH and osmolality, and provide essential gases (O2 and CO2). The ‘food’ portion of the culture medium consists of amino acids, vitamins, minerals, and carbohydrates. These allow the cells to build new proteins and other components essential for growth and function as well as providing the energy necessary for metabolism.
The growth factors and hormones help regulate and control the cells’ growth rate and functional characteristics. Instead of being added directly to the medium, they are often added in an undefined manner by adding 5 to 20% of various animal sera to the medium. Unfortunately, the types and concentration of these factors in serum vary considerably from batch to batch. This often results in problems controlling growth and function. When growing normal functional cells, sera are often replaced by specific growth factors.
The medium also controls the pH range of the culture and buffers the cells from abrupt changes in pH. Usually a CO2-bicarbonate based buffer or an organic buffer, such as HEPES, is used to help keep the medium pH in a range from 7.0 to 7.4 depending on the type of cell being cultured. When using a CO2-bicarbonate buffer, it is necessary to regulate the amount of CO2 dissolved in the medium.
This is usually done using an incubator with CO2 controls set to provide an atmosphere with between 2% and 10% CO2 (for Earle’s salt-based buffers). However, some media use a CO2– bicarbonate buffer (for Hanks’ salt-based buffers) that requires no additional CO2, but it must be used in a sealed vessel (not dishes or plates).
Finally, the osmolality (osmotic pressure) of the culture medium is important since it helps regulate the flow of substances in and out of the cell. It is controlled by the addition or subtraction of salt in the culture medium. Evaporation of culture media from open culture vessels (dishes, etc.) will rapidly increase the osmolality resulting in stressed, damaged or dead cells. For open (not sealed) culture systems, incubators with high humidity levels to reduce evaporation are essential.
Thing # 9. How to Decide if Cultured Cells are “Happy”
Evaluating the general health or “happiness” of a culture is usually based on four important cell characteristics: morphology, growth rate, plating efficiency and expression of special functions. These same characteristics are also widely used in evaluating experimental results.
The Morphology or cell shape is the easiest to determine but is often the least useful. While changes in morphology are frequently observed in cultures, it is often difficult to relate these observations to the condition that caused them. It is also a very difficult characteristic to quantify or to measure precisely.
Often, the first sign that something is wrong with a culture occurs when the cells are microscopically examined and poor or unusual patterns of cell attachment or growth are observed. When problems are suspected, staining the culture vessels with crystal violet or other simple histological stains may show growth patterns indicating a problem.
Cell counting and other methods for estimating cell number, on the other hand, allow the determination of the growth rate, which is sensitive to major changes in the culture environment. This allows the design of experiments to determine which set of conditions (culture media, substrate, serum, plastic-ware) is better for the cells, i.e., the conditions producing the best growth rate.
These same or similar techniques can also be used to measure cell survival or death and are often used for in vitro cytotoxicity assays. Plating Efficiency is a testing method where small numbers of cells (20 to 200) are placed in a culture vessel and the number of colonies they form is measured.
The percentage of cells forming colonies is a measure of survival, while the colony size is a measure of growth rate. This testing method is similar in application to growth rate analysis but is more sensitive to small variations in culture conditions. The final characteristic, the expression of specialized functions, is usually the most difficult to observe and measure.
Usually biochemical or immunological assays and tests are used. While cultured cells may grow very well in suboptimal conditions, highly specialized functions usually require near perfect culture conditions and are often quickly lost when cells are placed in culture.