ADVERTISEMENTS:
Cell Cloning:
In the traditional culture techniques, the cells are heterogenous in nature. Isolation of pure cell strains is often required for various purposes.
Cell cloning broadly involves the processes connected with the production of a population of cells derived from a single cell. Cloning of continuous cell lines is much easier compared to that of the primary cultures, and finite cell lines.
There are certain limitations for cloning of culture cells derived from normal tissues. These cells survive for a limited number of generations, and therefore cloning may not result in any significant number of cells. On the other hand, cloning of continuous cell lines due to their transformed status is much easier. Thus, the transformed cells have higher cloning efficiency compared to normal cells.
ADVERTISEMENTS:
Cloning may be carried out by two approaches- monolayer and suspension cultures:
1. Monolayer culture:
Petri dishes, multi-well plates or flasks can be used for cloning by monolayer culture. It is relatively easy to remove the individual colonies of cells from the surfaces where they are attached.
2. Suspension culture:
ADVERTISEMENTS:
Cloning can be carried out in suspension by seeding cells into viscous solutions (Methocel) or gels (agar). As the daughter cells are formed in suspension, they remain intact and form colonies in suspension.
Dilution Cloning:
Dilution cloning is the most commonly used technique for cloning of monolayer cells, and involves the following stages (Fig 39.1).
1. Trypsinization of cells (at log phase) to produce single cell suspensions.
2. Dilution of the cells to about 10-100 cells/ ml.
3. Seed the cells in multi-well dishes, petri dishes or plastic bottles.
4. Incubate under appropriate conditions for 1-3 weeks.
5. Isolate individual colonies.
The clones can be isolated directly from the multi-well dishes (by trypsinization), by cloning ring technique from petridishes, and by irradiating the plastic bottle.
Stimulation of Plating Efficiency:
ADVERTISEMENTS:
Plating efficiency represents the percentage of cells seeded at subculture that gives rise to colonies. The plating efficiency and cloning efficiency are said to be identical, if each colony is derived from a single cell. The plating efficiency is around 10% for continuous cell lines, while for primary cultures and finite cell lines, it is quite low — 0.5 to 5% or sometimes even zero. Several attempts are made to improve the plating efficiency in the culture laboratories. These approaches are based on the assumption that the cells at low densities require more nutrients and/or growth factors.
The stimulation of plating efficiency with regard to culture factors, conditioned medium and feeder layers is briefly described:
Culture factors:
Medium:
ADVERTISEMENTS:
A medium rich in various nutrients is better suited for improving the plating efficiency.
Serum:
Fetal bovine serum is better than horse or calf serum, if the addition of serum is required.
Addition of metabolites:
ADVERTISEMENTS:
Supplementing the medium with intermediary metabolites (e.g. pyruvate, α-ketoglutarate) and nucleosides stimulates plating efficiency.
Addition of hormones:
Dexamethasone, a synthetic analogue of hydrocortisone, improves plating efficiency e.g. fibroblasts, melanoma cells, chick myoblasts. Insulin also stimulates plating efficiency of several cell types.
Carbon dioxide:
ADVERTISEMENTS:
CO2 significantly influences plating efficiency. Most of the cells require 5% CO2 while for some cells, a lower CO2 concentration (around 2% CO2) is better e.g. human fibroblasts. HEPES is used to protect the medium from pH fluctuations.
Pretreatment of substrate:
The plates pretreated with fibronectin or polylysine show an improved plating efficiency.
Use of purified trypsin:
Some workers prefer to use purified trypsin instead of crude trypsin for trypsinization so that the plating efficiency is better.
Conditioned medium:
ADVERTISEMENTS:
A medium that has already been used for the growth of other cells contains certain metabolites, growth factors and other products that stimulate growth. Such a medium, referred to as conditioned medium, when added to cell cloning medium improves plating efficiency.
Feeder layers:
A layer growth-arrested living cells, referred to as feeder layer, promotes plating efficiency. This is because the feeder cells provide nutrients, growth factors and matrix constituents that support survival, growth and proliferation of cells.
Suspension Cloning:
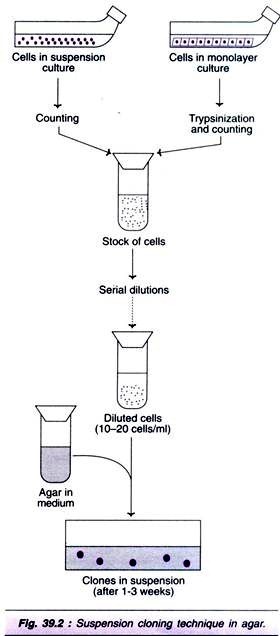
Certain cells, particularly the transformed fibroblasts and hematopoietic stem cells, can be more conveniently cloned in suspension rather than monolayers. Suspension cloning can be carried out by using agar or Methocel, which can hold the cells of a given colony together, and prevent mixing of colonies. The technique of cloning in agar suspension is carried out in the following stages (Fig 39.2).
1. Cells from suspension culture or monolayers can be used. The monolayer cells have to trypsinized while the suspension cells can be directly used.
ADVERTISEMENTS:
2. Count the cells and dilute serially so that 10-200 cells/ml are finally present.
3. Freshly prepared agar medium with appropriate dilution is used.
4. The agar medium is inoculated with the diluted cells.
5. Incubation for 1-3 weeks given clones in culture.
Isolation of Clones:
After the cloning is complete, selection and isolation of specific cell strains is the next important step. This is essentially required for the propagation of cells. If the monolayer cells are cloned directly in the multi-well plates, the colonies can be isolated by trypsinization of the individual wells. However, when the cloning is carried out in petri dishes, the colonies can be separated from the medium by placing stainless steel or ceramic rings around the colonies.
Micromanipulation:
The genuineness of a colony that is the colony is derived from a single cell can be done by micromanipulation. This can be achieved by monitoring the colony formation at the early stages of cell cloning. Micromanipulation, if properly done, is believed to be the conclusive method for determining the genuine clonality of a clone.