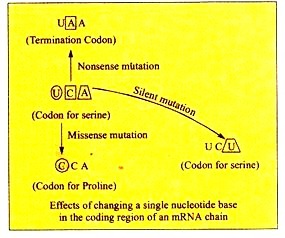
ADVERTISEMENTS:
1. Substrate Concentration Effects:
Enzyme activity as measured by the rate of product formation increases as a hyperbolic function as the substrate concentration is raised (Fig. 11-3) until a maximum reaction velocity (Vmax) is achieved.
Excessively high substrate concentrations may actually reduce enzyme activity.
Each enzyme, subject to experimental conditions, has a characteristic Vmax as exemplified in Table 11-1 for the glycolytic enzymes in brain tissue.
If the substrates present in brain tissue were to occur in excess, one would expect that the reaction catalyzed by aldolase (which has the lowest Vmax) would be the rate- limiting reaction in the sequence.
However, in vivo studies indicate that enzymes are rarely saturated by substrate. Under typical conditions, hexokinase, phosphoglucoisomerase, and aldolase operate in the presence of substrate concentrations equal to or somewhat greater than the KM value (Michaelis-Menten constant).
Small changes in substrate concentration do not significantly alter the rate of metabolism in this part of the glycolytic pathway. Of greater regulatory importance for these reactions is the amount of enzyme present. The last six glycolytic enzymes in brain tissue operate at substrate levels that are significantly below the KM value.
ADVERTISEMENTS:
Therefore, small changes in substrate concentration would be more likely to alter the rates of their respective reactions. Under anoxic conditions, the rate of production of lactate is not appreciably increased by supplementing the tissue with glucose. However, supplements of compounds such as glycerol, which enter the glycolytic pathway below the level of adolase, cause an increase in lactate production.
The affinity between enzymes and their substrates affects the rates of enzyme-catalyzed reactions. For example, at branch points of metabolic pathways, two enzymes compete for the same substrate. The reaction catalyzed by the enzyme with the lower KM value is favored at low substrate concentrations whereas the reaction catalyzed by the enzyme with the higher KM value is favored at high substrate concentrations (Fig. 11-4).
Thus, when present in low concentrations, a substrate may be channeled primarily into one pathway, whereas the major direction of metabolism may shift to other pathways at higher substrate concentrations.
2. Effects of Salts and pH:
A variety of environmental factors influence the structure of proteins and as such influence the catalytic activity of enzymes. Small changes in pH or an increase (or decrease) in the salt content of the enzyme’s environment may quickly be followed by a dramatic change in the level of activity of the enzyme. A change in activity of one enzyme of a pathway will change the activity of the metabolic pathway as a whole.
3. Covalent Bond Modification:
A number of enzymes are produced in an inactive or zymogen form and must be modified through cleavage of certain covalent bonds in. order to become active. The modification may be irreversible, as is the case with hydrolytic modification of zymogens such as pepsinogen (to form pepsin) and trypsinogen (to form trypsin).
Other enzymes may be reversibly covalently activated and deactivated. The reversible activation and deactivation of glutamine synthetase is a well-studied example (Fig. 11-5). This enzyme catalyzes the conversion of glutamate to glutamine,
Glutamine synthetase is inactivated by a reversible adenylation in which 12 molecules of AMP become covalently bonded to the hydroxyl groups of 12 tyrosine residues of the enzyme. The inactivation is catalyzed by adenylating enzyme, which uses ATP as the source of adenylate and releases pyrophosphate. Reactivation is brought about through the action of deadenylating enzyme, which, in the presence of inorganic phosphate, cleaves the bonds linking the AMP molecules to the inactive form of the enzymes, thereby producing ADP (Fig. 11-5).
4. Isozymes:
In a number of instances, an enzyme that catalyzes a specific reaction may exist in multiple forms- in a cell or organism. These multiple forms or isozymes have different chemical structures, and can therefore be separated from one another by electrophoresis and chromatographic procedures.
Although they catalyze the same reactions, the isozymes usually have different KM and Vmax values and catalyze the reaction more or less effectively depending on the reaction conditions. One of the most exhaustively studied of the known isozymes is lactic dehydrogenase, which catalyzes one of the terminal reactions in glycolysis, that is, pyruvate + N ADH + H + <===> lactate + N AD +
In rats and in a number of other vertebrates, this enzyme is present in five forms. Each of the five forms has a molecular weight of about 134,000, consists of four polypeptide chains of about 33,500 each, and catalyzes the same reaction.
Each of the four polypeptides may be of two types, usually referred to as M and H. In rat skeletal muscle tissue, the predominant form of the isozyme contains four polypeptides of the M type; in contrast in rat heart muscle, the predominant form contains four polypeptides of the H type.
ADVERTISEMENTS:
The other isozyme forms are made up of three H and one M, two H and two M, and one H and three M polypeptides (usually abbreviated H3M, H2M2, and HM3). Some of the latter forms predominate in other tissues, although each tissue has some of each isozyme (Fig. 11-6).
The M4 isozyme is prevalent in embryonic tissue and in skeletal muscle tissue. It has a low KM and a high Vmax. It is well adapted to these tissues, which are frequently deprived of oxygen and must depend on the energy made available during the breakdown of glucose to lactate. Similarly, the H4 isozyme is well suited to the metabolism of heart muscle.
This isozyme has a high Km and a low Vmax and is inhibited by excess pyruvate. Heart muscle is primarily aerobic, converting pyruvate to CO2 and H2O rather than to lactate. The lactic dehydrogenase enzyme is most active during emergency conditions when the oxygen supply is low.