ADVERTISEMENTS:
The following points highlight the top sixteen techniques used in cell biology. Some of the techniques are: 1. Immunofluorescence Microscopy 2. Ion-Exchange Chromatography 3. Affinity Chromatography 4. Partition and Adsorption Chromatography 5. Gel Filtration Chromatography 6. Radioactive Tracer Technique 7. Radioimmunoassay (RIA) 8. Enzyme Immunoassay 9. Spectroscopy and Others.
Cell Biology: Technique # 1.
Immunofluorescence Microscopy:
Immunological technique is the method to locate an antigen to a particular position of the cell of specific antibody for a particular protein to be studied. Immunofluorescence is another slightly modified technique used to study cells under fluorescence microscopy to locate the distribution of the antigen in the cells.
In this case the first antibody is un-labelled and the second antibody is made against IgGs of the organisms in which the first antibody is made. This secondary antibody is coupled to some fluorochrome such as Fluorescein Isothiocyanate, Rhodamine etc. This method is used to study cellular architecture, subcellular localisation and the localisation of specific proteins during cellular activities and cell cycle events.
ADVERTISEMENTS:
The microfilaments, microtubules and intermediate filaments can be studied to have an idea about the cytoskeletal structure of the cell (Fig. 8.1). This technique is useful to study many structures at higher resolution in the electron microscope top.
Antibodies to many cellular proteins are available in the market from different firms like Amershain, Dako, Sigma etc. Primary antibodies may be monoclonal or polyclonal. Secondary antibodies are generally coupled with fluorochrome for immunofluorescence studies. Antibodies can be stored at -70°C or at — 20° C.
The main instrument needed for the immunofluorescence studies is the fluorescence microscope with automatic photomicrograph system. Confocal microscopy is being recently used for detailed studies of the structures in or near the nucleus and also for round cells to obtain greater resolutions at different levels in the cell.
Cell Biology: Technique # 2.
Ion-Exchange Chromatography:
In this method, molecules are separated on the basis of differences in charge. Many biological macromolecules, such as amino acids and proteins, have ionisable groups. They may carry positive or negative charge. The charge showed by these compounds depend on the pH of the solution.
ADVERTISEMENTS:
The ion-exchange separations are performed in columns packed with ion-exchanger. Two types of ion-exchangers are present like Cation and Anion exchangers. Cation exchangers are negatively charged and so they can attract positively charged molecules.
Anion exchangers are positively charged, so they can bind negatively charged molecules. Tire commercial ion-exchangers are made of porous polystyrene beads. The co-polymerisation of styrene is made with varying proportions of divinylbenzene and styrene.
The most common example is Dowex 50, Sephadex etc. Sometimes chemically modified celluloses are used instead of polystyrene-based exchangers, such as DEAE-cellulose, Carboxy-methyl cellulose etc.
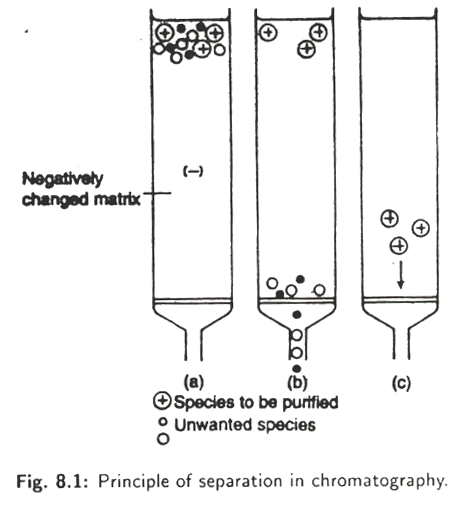
When the samples to be separated are passed through a column, molecules with opposite charge will bind while the other molecules with the same charge of the medium used in the column and some unwanted materials will pass through (Fig. 8.1).
As the desired compound is retained in the column, this technique is sometimes known as Sorption chromatography. The bind molecules can be eluted by increasing the concentration of the buffer or by changing the pH of the buffer. Sometimes the unwanted molecules are also retained within the column—thus eluting the desired substances.
Some examples of ion- exchangers used in biology have been shown in Table 8.1.:
Ion-exchange chromatography has application in separating amino acids and proteins. The selection of strong and weak exchanger depends on the stability over pH and the effect of pH on charge. Generally, cationic buffers like Tris, Pyridine and Alkyl amines are used with anion exchangers and anionic buffers like Acetate, Barbiturate and Phosphate are used with cation exchangers.
ADVERTISEMENTS:
Two types of elution can be made, i.e., by passing the single buffer throughout the separation which is known as Isocratic separation. When the gradient of buffer is passed through the column it is called Gradient elution.
The separation of amino acids is generally performed using strong acid cation exchanger. Gradient elution method helps in the sequential elution of amino acids. The acidic amino acids come out first followed by the neutral amino acids like Glycine and Valine.
This is followed by basic amino acids like Arginine and Lysine. This principle is also followed in the Amino acid Analyser. Proteins are separated generally through weakly acidic or basic exchangers. The elution of proteins takes place on the basis of their isotonic points.
Cell Biology: Technique # 3.
Affinity Chromatography:
In this method, the property of biological interactions between the molecules is used in order to get separation and purification. In this case some ligand molecule (i.e., substrate of an enzyme) or some antibody is bound to the matrix of the column.
ADVERTISEMENTS:
On passing the molecules through the column, only those materials that form a specific bond or complex with the ligand will be retained and all others will pass through the column (Fig. 8.2).
The main principle of this method is that the compound to be purified is passed through the column containing some immobilised ligand, and the desired compound will bind to the ligand. The ligand is the substrate in case of enzymes.
Some of the ligands used in this method are shown in the Table 8.2.:
Instead of column, this method can be utilised on nitrocellulose membranes for the purification of single-stranded DNA molecules. When the enzyme is to be purified, the ligand used is generally the substrate and, for the separation of proteins, specific antibodies are used.
The bound-molecules can be eluted by increasing the ionic strength of the buffer. Thus this type of chromatography is highly specific and versatile. This affinity chromatography is very useful for the purification and separation of macromolecules of very small amount.
The mRNA is purified and isolated from various types of RNA through Affinity chromatography. Immobilised single-stranded DNA is used to isolate complementary RNA or DNA.
Cell Biology: Technique # 4.
Partition and Adsorption Chromatography:
It is a common practice to separate many substances by shaking the substance in two immiscible liquid phases in a separating funnel. When a substance is shaken in the solvent it will partition with the formation of two phases. If one phase is allowed to move the substance will also move on the basis of its partition coefficient.
ADVERTISEMENTS:
The substance will move rapidly if it likes the mobile phase while, if it prefers the stationary phase, it will move slowly. Now the mobile phase may be liquid or gas and, if the substance is adsorbed on the stationary phase and starts to move along with the stationary phase, then it is called Adsorption chromatography.
The substance will move at a varying speed depending on the intensity and characteristics of adsorption and solubility in the solvent used for separation. Adsorption chromatography can be performed either in the column or on the thin layer of the matrix. Adsorbents may be Silica gel, Aluminum oxide, Calcium carbonate, Magnesium carbonate, cellulose etc. which are used as the stationary phase.
Hydroxyapatite (Calcium phosphate) is used in the column to separate proteins, nucleic acids etc. It has the unique property of binding double-stranded DNA and not single- stranded DNA. For mobile phases, different organic solvents may be used, depending on the polarity of the compounds to be resolved.
The basic principle of Adsorption and partition chromatography is used in Paper chromatography, Thin-layer chromatography and Gas chromatography. In case of paper chromatography, the paper is the support. Water is used to moist the paper and this hydrated phase is the stationary phase. The organic solvent which is used in the chromatography is the mobile phase which moves rapidly through the aqueous phase.
In Gas chromatography, the partitioning takes place between a liquid and a gas phase. Here the gas is the mobile phase and the nonvolatile liquid that coats the matrix substances of a column is the stationary phase.
This partitioning depends on the temperature and the gradual increase in temperature helps more and more of the substance to come out in the mobile gas phase from the stationary phase. This method is widely used for the qualitative and quantitative analyses of large number of compounds.
ADVERTISEMENTS:
The principle for the separation is the difference in the partitioning of the volatilized compounds between the liquid and gas phases. During the passage of the substance through the column a detector is attached with a chart recorder, which scan the peak as the substance passes through the detector.
Cell Biology: Technique # 5.
Gel Filtration Chromatography:
This type of chromatographic separation takes place on the basis of the size and shape of molecules utilizing the porosity of the gel materials. This method is also known as exclusion or permeation chromatography.
This is done by using a column full of matrix consisting of Sephadex, Agarose, Sepharose, Bio Gel A, Bio Gel P, Polystyrenes (Bio-beads S) etc. Varying properties of the matrix axe obtained by crosslinking the poly-dextran with other compounds.
Most of the gel compounds are hydrophilic in nature, thus causing very little-denaturation and adsorption of sensitive biochemical substance. Sephadex and polyacrylamide gels are used as swollen beads. Many gels are available in the market as Superfine, Fine, Medium and Coarser.
The coarser bead shows fast flow rate but with poorer resolution. Fine and Superfine beads are used for analytical work and the coarse one for preparative work.
ADVERTISEMENTS:
When the molecules of different sizes and pores are passed through a column, molecules larger than pores of gel will pass through it. Molecules of small pore size will enter the beads and flows are retarded in the column.
The main use of this procedure is in the purification of macromolecules, viruses, proteins, enzymes, hormones, antibodies, nucleic acids, amino acids etc. It is also used for the isolation of ribosomal proteins.
Cell Biology: Technique # 6.
Radioactive Tracer Technique:
The easiest method for monitoring cellular events and their localisation in the cell is the use of radioactive isotopes. The most commonly used isotopes in the biological research are H3, C14, P32, S35, I125, I131. They release high energy electron or beta particles during their radioactive decay.
Radioactive decay is a spontaneous process and its rate varies with the source. The number of atoms disintegrating at any time is proportional to the number of atoms present in the isotope at that time. Conveniently, it is expressed as half-life which is defined as the time taken for the activity to fall from any value to half that value. The half- life of some important isotopes is shown in the table 8.3.
According to SI system, the unit of radioactivity is Becquerel (Bq), which is one disintegration per second. But the most commonly used unit is the curie (Ci) which is measured as the number of nuclear disintegrations per second as compared to that of 1 gm of Radium, i.e., 3.7 x 1010 per second.
In case of biological materials, the micro curie (µCi) and mill curie (mCi) are used. The disintegrations measured by the Counter are referred to as Counts.
For any biological research, macromolecules of the cell are made radioactive by administering the radioactive compounds to tissues or cells and then the fate of the radioactive compound can be monitored. For the study of DNA synthesis in the various types of cells or tissues, the use of H3 is necessary.
Many organic and inorganic compounds labelled with Tritium are available of Bhabha Atomic Energy Research Centre, Mumbai. Tritiated Thymidine and Uridine are used for the study of DNA and RNA, respectively.
The radioactive substance is added in the solution where cells or tissues are exposed for a certain period of time, either cells are fixed at regular intervals or aliquots are removed at various time intervals and the radioactivity is noted.
The radioactivity may be measured through Liquid Scintillation Counter or the location of the radioactivity in different positions of the cell, can be noted through Radioactive Tracer Technology, called Autoradiography. In this method labelled cells are fixed and squashed on a slide or can be spread on a slide.
Then a thin layer of Special Auto radiographic stripping film is placed over it and is kept in the dark for exposure. Instead of film, sometimes the slide is coated with photographic emulsions for autoradiography and is kept in the dark.
During storage of slides in the dark, the emission of beta particles from the radioactive substance activates Silver halide crystals of film or emulsion. After a few weeks, the slides are developed like the photographic film, which show the activated silver crystals as black spots under the light microscope.
The auto radiographic technique is also applicable to electron microscopy where cells or tissues are placed on a grid instead of a slide. Here Silver grains are found to be opaque and electron dense against the electron transparent background.
The important use of this radioactive tracer technique is to note the metabolic pathway in the cell using Pulse-chase experiment through some radioactive precursor. When tissues or cells are exposed to some radioactive compounds for a certain period of time, it is called Pulse and then cells are kept in a radioactive free medium after washing is called Chase.
By fixing cells or taking aliquots from cell fractionation in different times, biochemical transformations and movement of the precursor (labelling) can be observed in the cell through autoradiography or through Scintillation Counter.
Cell Biology: Technique # 7.
Radioimmunoassay (RIA):
This method is widely used in Biochemistry and also in clinical fields for diagnostic purposes. The quantitative analysis of hormones, steroids and drugs can be done with the help of this method. As the name implies, it combines the method of immunology as well as the radioisotope labelling techniques.
In this method labelled antigen, un-labelled antigen and fixed quantity of antibody are mixed together and a calibration curve is plotted with percentage of labelled antigen against the un-labelled antigen added (Fig. 8.3).
Un-labelled antigen is treated as samples. Then antibody-bound antigen is separated from free antigens through ion-exchange or adsorption chromatography. Sometimes the mixture of these antigens and antibodies are passed through Sephadex column, when the bound antibodies remain attached to the Sephadex beads and the unbound antigens can be washed out.
Then the bound labelled antigens are eluted from the column and can be quantitatively arranged through Liquid Scintillation counter. Antibodies are generally labelled with H3,C14 or I131. When cells or tissues axe used, radiolabeled antibodies can be used with the help of autoradiography to localize the various components within the cell.
Cell Biology: Technique # 8.
Enzyme Immunoassay:
This method is also known as enzyme-linked immunosorbent assay or ELISA—it combines the principle of Antibody-antigen reactions as well as the spectrophotometric enzyme assays by using antibodies or antigens conjugated with some enzymes like Alkaline phosphatase, Alcoholic dehydrogenase, etc. ELISA is easy to operate and the cost is also less. Hence ELISA is gradually replacing RIA (Fig. 8.4).
In this method, specific antibodies are attached to solid phase (filter paper, polystyrene micro titration plates etc.) then a limited amount of labelled antigen (with a specific enzyme) and excess amount of un-labelled antigen were added, incubated and washed.
Some un-labelled antigens will also attach with antibodies. The enzyme substrate is then added and the enzyme activity is measured through spectrophotometer. The amount of enzyme activity is found to be directly proportional to the amount of antigen present. The sensitivity of ELISA is greatly enhanced through enzyme amplification technique using double antibody method.
ELISA is used in clinical fields to measure any antigen, immunoglobin, haematological factor, hormone, etc. It is also used in the detection of bacterial toxins, viruses, Hepatitis B surface antigen etc. and in the assay of different antibodies like antiviral and antifungal antibodies.
Instead of enzymes, sometimes some fluorochrome is tagged with antibody to assay the-antigens and antibodies using fluorescence system. Then this method is known as Fluorescence Immunoassays (FIA).
Cell Biology: Technique # 9.
Spectroscopy:
Variety of techniques are developed with the principle of spectroscopy, i.e., study of interaction between electromagnetic radiation and the substance. Light, heat, microwaves, infra-red, X-rays etc. have electromagnetic waves with average speed of 3 x 108 m/sec.
These waves are composed of two components, such as electric field and magnetic field which are oscillating as perpendicular to each other. Light has both properties such as waves and particles.
Electrons of an atom remain in the lowest energy level in the ground state. When an atom is treated with light, then its electrons move from the ground state to the excited state with the absorption of light or electromagnetic radiation. When an excited electron returns to the ground state, it will emit radiation of certain specific wavelength (energy) which is utilised in spectroscopic analysis.
In some substance, emission of energy takes place spontaneously, i.e., without application of any external radiation. Besides absorption or emission of light, atoms of certain substances undergo some changes in electronic or nuclear properties when the molecule is exposed to electromagnetic radiation.
The study of absorption or emission properties as well as some changes in the nuclear structure gives many information of different macromolecules.
Cell Biology: Technique # 10.
Nuclear Magnetic Resonance Spectroscopy (NMR):
It is a method for detecting interaction between the nuclei of an atom with the magnetic field of the electromagnetic radiation. It is known that protons and neutrons of the atom have spin properties, When protons and neutrons of an atom are present in pairs in the nucleus, there will be no net spin.
But if there is any unpaired protons, these protons will impart a magnetic moment which can interact with an applied magnetic field, i.e., the nuclei will absorb the energy and may lie either in a low energy state (nuclear spin parallel with the field) or in a higher energy state (antiparallel to the field).
This interaction of unpaired proton with the magnetic field is the main principle of NMR spectroscopy. In a magnetic field, these nuclei absorb radiation of radio wave length showing a phenomenon known as nuclear magnetic resonance.
For NMR study, unpaired nuclei like H1, C13, N14, O17, p31 etc. are commonly used in case of biological materials. NMR spectra are plotted as energy absorbed against the magnetic field strength. 40 MHz radio wave frequency is used to obtain resonance of H1 nucleus. The NMR spectrometer scales in tan (T) units.
The basic NMR instrument includes:
1. Source of radiation,
2. Receiver to detect the absorption of energy,
3. Magnetic field,
4. Oscilloscope or Recorder.
NMR is used for the study of molecular structure of some organic molecules, action of different antibiotics and drugs on living systems, any alteration in the structure of molecules in the plasma membrane, effect of cholesterol on erythrocyte membranes etc. High field NMR instruments (750 MHz, qvo MHz) have been developed to explore the structure and dynamic properties of proteins in solution.
Cell Biology: Technique # 11.
Optical Rotatory Dispersion (ORD) and Circular Dichroism (CD):
The three-dimensional structure of macromolecules in solution can be studied by noting their properties of absorption of polarised light. The plane polarised light is a type of light consisting of waves oscillating in a single plane. This is obtained by passing a beam of light through a Nicol prism or a polarizing screen.
When a plane polarised light is passed through a substance, the polarised light will rotate to a certain angle depending on its structure. It has also been found that this depends on the wavelength of light. Hence, the rate of change of rotation is measured with wavelength of light which is known as Optical Rotatory Dispersion (ORD).
Certain optically active substances have been found to absorb polarised light differently, i.e., differential absorption of right (R) and left (L) circularly polarised light. Thus another spectroscopy—Circular Dichroism Spectroscopy (CD)—has been developed to investigate the interaction of polarised light and the samples.
Both CD and ORD are almost same but, due to the relative simplicity of CD spectra, CD analysis has gained its superiority. The resolution of CD bands is also superior.
Circularly polarised lights is obtained by superimposing two plane polarised light of the same wavelength which come through the monochromator and Nicol prism. This superimposed light can be resolved into Right (R) and Left (L) waves. Certain substances absorb differentially R and L waves and show refraction with elliptically polarised beam.
ORD and CD are useful in the study of the secondary structure of macromolecules, particularly protein and amino acids in solution. CD spectra is also useful for the study of binding of substrate and inhibitor to the enzyme.
The helical structure of DNA and protein can be studied with the CD spectra. CD spectrum, is very sensitive to any structural changes of the macromolecule. So, any interaction of protein with nucleic acids can be studied by observing changes in the CD spectra. The transitions between double-stranded and single- stranded nucleic acids can be studied with CD spectrophotometer.
Cell Biology: Technique # 12.
Infra-red (IR) Spectrophotometry:
Infra-red (103-104 nm) shows vibrational spectra, so molecules under infra-red show different vibrational levels. These vibrational levels change with the bonding characteristics of the compound. For example, vibrations of C – H,-CH2 and CH3 will differ.
Similarly, various functional groups like methyl, carbonyl, amide groups etc. will show different IR spectra. Hence the IR spectra is useful in biochemistry, particularly for the study of macromolecules and membranes, for the identification of drugs, for the study of secondary structure of proteins, such as the number of helical structures present in protein etc.
Cell Biology: Technique # 13.
Atomic Absorption/ Flame Spectrometry:
When vitalization of atoms of any compound occurs either in a flame or electro thermally, then it absorbs or emits atoms of specific wavelength. Emission flame spectrophotometer measures the emission of specific wavelength of atoms in a flame which is used to assay different elements present in any biological sample.
Atomic absorption spectrophotometer detects the absorption of a particular wavelength by atoms of a sample when it is heated either in a flame or otherwise. The flameless method is more sensitive than flame spectrophotometer. This atomic absorption spectrophotometer is useful in measuring amount of heavy metals or other toxic metals present in any biological sample.
The main components of the flame are:
1. Nebulizer or Atomizer which makes five drops of the sample in solution, and then pass these drops with a forced air pressure to the burner (flame).
2. Monochromator is used to select the wavelength.
3. Detector containing a photocell.
In case of Atomic absorption spectrophotometer, a source of white light (cathode discharge lamp) and a double monochromator are used in addition to a detector and an atomizer.
The sample is placed in a metal cup, high voltage and an anode carrier gas are used (Table 8.4.):
Cell Biology: Technique # 14.
Flow Cytometer:
Principle:
The instrument, Flow Cytometer, is based on the principle that single cells in suspensions axe passed through a field of illumination and each cell is quantitatively assayed by staining cells with fluorochromes or noting the scattering of light by each cell.
Procedure:
Cell suspensions are placed at first in a flow cell fitted with a device to form a liquid jet. Cells will travel through the center of a liquid jet at the rate of 5-10 m/second. Cells are then passed through the area of intense light. If the cells contain some fluorochromes, fluorescence emission can be detected using lens, beam splitters and photomultiplier tubes (Fig. 8.5).
Different fluorochromes used will show different types of fluorescence emission. These signals are then quantified and are kept in the memory of computers as histograms. During the flow, droplet formation is done using piezoelectric crystal in the flow cell.
At the end of the instrument there is a droplet deflector assembly. Giving a definite quantitative value to the computer for collecting a specific cell, desirable will then be collected in a microtiter plate or tube and undesirable cells will be collected in the waste. Different fluorochromes are used for different types of cells which will then help to sort the cells having two types of nuclei (heterokaryons).
Cell Biology: Technique # 15.
Applications of Flow Cytometer and Cell Sorter:
This instrument has a great importance in selecting somatic hybrid and cybrid cells using fluorescence labelling technique. It can also be applied to know the physical and physiological characteristics of plant protoplasts which has a great importance in molecular biology and in the manipulation of plant protoplasts. This instrument is also useful in the study of the cell cycle and in the isolation of chromosomes.
This is also suitable for cell sorting. In this case, the fluid-containing cells is charged temporarily and the charged cells of desirable particles axe deflected in the electric field. These are collected in the tube for the collection of mitochondria, nuclei, chromosomes besides cells, protoplasts and pollen.
Flow cytometer is also used to measure the DNA content of cell, chromosomes etc. for the measurement of the ploidy level in short time. In case of cell-fusion studies heterokaryons can be rapidly sorted out in flow cytometer. In Genetic Engineering studies, transformed or hybrid cells can be sorted out with this instrument.
Cell Biology: Technique # 16.
Non-invasive Scanning of Soft Tissues:
Several methods are now developed to examine the soft tissue of the body, particularly the brain, without injecting any colored substance in the body.
These include:
1. Computer Tomography Scan (CT Scan).
2. Magnetic Resonance Imaging (MRI).
3. Microscopic Magnetic Resonance Imaging (mMRI).
4. Positron Emission Tomography (PET).
In CT scan, differences in the absorption of X-ray by the brain tissue are used to construct a three dimensional image (3D image). Here the dose of X-ray needed is very low.
The principle of MRI is to use magnetic field which shows differences in vibration of Protons (HT) of the water molecules present in the tissues of body. This vibration of water molecule depends on the chemical surroundings of the tissue. Besides protons of water molecules, the vibration of other atoms like Flurosive, Sodium, Phosphorus, Nitrogen can also be detected.
Through MRI studies, many things of the metabolic processes of brain and other organs may be investigated. Both these types of scanning help to detect the location of tumours, sites of hemorrhage, intracranial bleeding etc. MRI is better than CT scan because it does not require X-ray. Again, all these modern techniques are possible only with the help of computer.
mMRI is the most technically complex of all techniques. It requires placing the specimen inside a strong magnetic field. The advantage of mMRI is that it can image specimen which are too large and opaque and it can also image living specimen. It can also provide digitally recording anatomical information from intact specimen. Unique contrast mechanism can be applied to highlight the different features of specimens.
PET scan is based on the use of positron, which is like an electron except that it has positive (+) charge. It also gives 3D image where the molecules are located. It helps to measure the blood flow in the brain, glucose utilisation and oxygen consumption, and for the diagnosis of psychiatric disorders, brain tumours, epilepsy and le-generative changes due to Alzheimer’s disease.