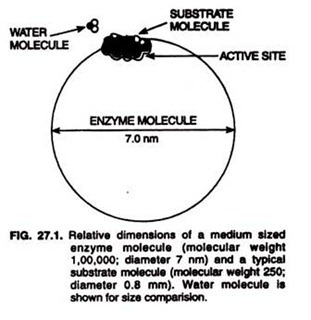
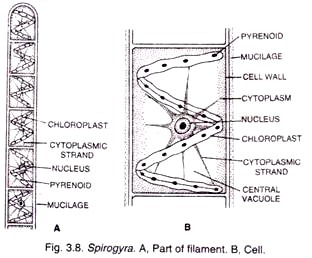
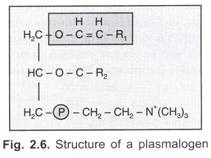
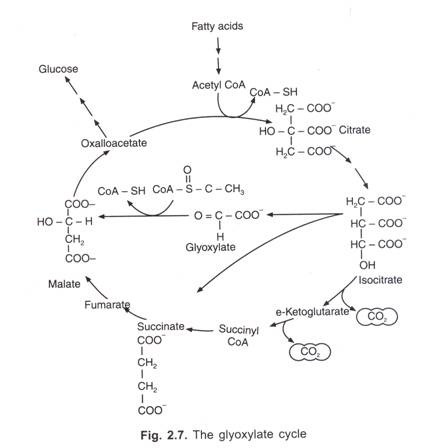
ADVERTISEMENTS:
Some of the most important types of special compounds found in cell metabolism are as follows:
1. Nucleoside Phosphates 2. Cyclic Nucleoside Monophosphates 3. Pyridine Nucleotides.
Among the compounds that have special roles in cell metabolism are the nucleoside phosphates (e.g., adenosine triphosphate, ATP; adenosine diphosphate, ADP; and adenosine monophosphate, AMP), the pyridine nucleotides (e.g., nicotinamide adenine dinucleotide, NAD+; and nicotinamide adenine dinucleotide phosphate, NADP +), and the vitamins.
ADVERTISEMENTS:
1. Nucleoside Phosphates:
The metabolic activities of cells are accompanied by a complex series of energy transformations, and the principal intermediaries of these energy exchanges are the nucleoside phosphates. Although ATP (Fig. 3- 8) serves as the immediate energy donor in most energy-consuming (i.e., endergonic) reactions, other nucleoside triphosphates such as guanosine triphosphate (GTP), uridine triphosphate (UTP), and cytidine triphosphate (CTP) serve in this capacity in certain instances.
Energy-producing (i.e., exergonic) reactions are often accompanied by the synthesis of nucleoside triphosphates from the corresponding nucleoside diphosphate.
The high rate of turnover of ATP attests to its vital role in metabolism. An examination of the chemical structure of ATP (Fig. 3-8) sheds some light on why this molecule so readily serves as an energy source for energy-consuming cellular reactions. The three phosphate groups of ATP exist in an ionized state at physiological pH and the four negatively charged oxygen atoms strongly repel one another. When ATP is hydrolyzed,
ADVERTISEMENTS:
ATP4- +H2O – ADP3- + HPO4
both of the products are negatively charged and continue to repel each other. The amount of energy that must be consumed in order to recombine these anions to re-form ATP reflects the amount of energy that is made available during the hydrolysis. For many years, the bonds linking successive phosphate groups in ATP were represented by a “squiggle” (i.e., ~ P) and called “high-energy bonds.”
Actually, there is nothing special about the bonds themselves and the amount of bond energy is not that great (31 kJ or 7.3 kcal per mole). What is important is that the phosphate groups have a very high transfer potential. The study of energy transfer and transformation is a special field of biochemistry called bioenergetics.
2. Cyclic Nucleoside Monophosphates:
AMP and GMP have cyclic forms called cyclic AMP (cAMP) (Fig. 3-9) and cyclic GMP (cGMP). Both cAMP and cGMP have been found to have a regulatory influence in a variety of cellular processes. cAMP is produced in the plasma membrane of cells and acts as an intracellular transmitter (sometimes called a “second messenger”) by triggering certain metabolic reactions in the cell.
The production of cAMP in the plasma membrane takes place as a response to compounds (usually hormones) that bind to specific receptor sites on the outside surface of the membrane. A good example of the regulatory role played by the cyclic nucleotides is the action of cAMP during periods of exercise in which great demands are made by the muscle tissues for free sugars. During exercise, the hormone epinephrine is released into the bloodstream and on reaching the liver and muscles binds to the plasma membrane. cAMP formed in the membranes in response to epinephrine binding then diffuses into the cytosol.
In the cytosol, cAMP binds to and activates an enzyme called protein kinase, which in turn catalytically activates a second enzyme, phosphorylase. Phosphorylase initiates the breakdown of glycogen, thereby making available the large numbers of free sugar molecules whose consumption fulfills the energy demands of muscular activity.
ADVERTISEMENTS:
cAMP also influences transcription of nuclear DNA where it binds to the catabolite gene activator protein (CAP), and in the case of the lac operon promotes the transcription of genes for specific cellular enzymes.
Both cAMP and cGMP appear to be involved in the control of cell proliferation. Cells that are in the log phase of growth contain smaller amounts of cAMP and higher amounts of cGMP than cells in other phases of the growth cycle. cAMP and cGMP affect the assembly of microtubules in cells and this would be expected to directly influence cell growth.
3. Pyridine Nucleotides:
The pyridine nucleotides NAD+ and NADP+ usually function as coenzymes for certain dehydrogenation reactions in cells. The hydrogens that are removed from compounds by various dehydrogenase enzymes are accepted by the pyridine nucleotides. Hydrogens are usually removed in pairs, with one hydrogen binding to the coenzyme as a hydride (Fig. 3-10) and the other carried as a hydrogen ion.
Vitamins:
Vitamins are organic substances that are present in small amounts in all cells and are essential to certain phases of the cell’s metabolism. Although vitamins are not used in large amounts by cells, the lack of a particular vitamin is usually accompanied by characteristic deficiency symptoms. Not all organisms are able to synthesize all of the vitamins that are essential to their metabolism, and they therefore rely on an external dietary source.
The role of most vitamins is closely allied to enzyme function, the vitamin comprising all or part of the non-protein portion of the enzyme (called the coenzyme). Table 3-10 lists the more common vitamins, the coenzymes of which they are a part, and the role of the coenzymes and/or the associated enzyme. The vitamin niacin is required for the synthesis of the coenzymes NAD+ and NADP+ described above (Fig. 3-10).
Riboflavin (vitamin B12) is used in the synthesis of the coenzyme flavin adenine dinucleotide (FAD), which also serves as a hydrogen acceptor in dehydrogenation reactions. The vitamin pantothenic acid is used in the synthesis of coenzyme A. The vitamin constituents of these two coenzymes are shown in Figure 3-11.
Unlike animals, which rely on an exogenous source of certain vitamins, plants are able to synthesize all their required vitamins; most of the synthesis takes place in the plant’s green tissue.