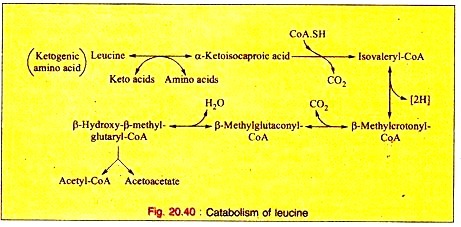
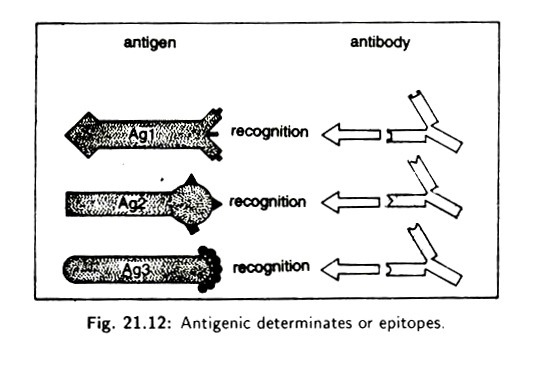
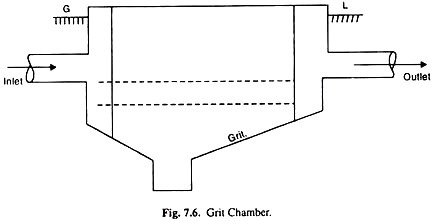
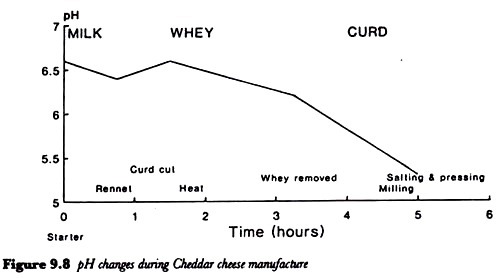
ADVERTISEMENTS:
We will be concerned with the organization, composition, and assembly of the cytoplasmic ribosomes of prokaryotic and eukaryotic cells.
The ribosomes present in cytoplasmic organelles (e.g., chloroplast and mitochondrial ribosomes). Although functionally analogous, many differences exist between the ribosomes of prokaryotic and eukaryotic cells (Table 22-2).
Considerably more is known about the structure and composition of bacterial ribosomes than ribosomes of eukaryotic cells, as will become evident during the discussion that follows. Most of the work on prokaryotic ribosomes has been carried out using Escherichia coli. Although some variations are observed among the prokaryotes, findings using E. coli are generally representative.
Ribosomes in the cytoplasm of eukaryotic cells have a sedimentation coefficient of about 80 S (MW about 4.5 x 106) and are composed of 40 S and 60 S subunits. In prokaryotic cells, ribosomes are typically about 70 S (MW about 2.7 x 106) and are formed from 30 S and 50 S subunits.
The complete ribosome formed by combination of the subunits is also referred to as a monomer. Although ribosomes from both prokaryotic and eukaryotic sources are about 30 to 45% protein (by weight), with the remainder being ribonucleic acid, the specific protein and RNA components of these two major classes of ribosomes differ (Table 22- 2 and Fig. 22-1); carbohydrate and lipid are virtually absent.
Magnesium ions (and perhaps other cations) play an important role in maintaining the structure of the ribosome. Dissociation into subunits occurs when Mg2+ is removed. The precise role (or roles) of Mg2+ remains uncertain, although interaction with ionized phosphate of subunit RNA is presumed.
Prokaryotic Ribosomes:
ADVERTISEMENTS:
RNA Content The small subunit of prokaryote ribosomes contains one molecule of an RNA called 16 S RNA (MW 0.6 x 106), and the large subunit contains two RNA molecules, a 23 S RNA (MW 1.1 x 106) and a 5 S RNA (MW 3.2 x 104) (see Table 22-2). All three rRNAs are products of closely linked genes transcribed by RNA polymerase in the sequence 16 S 23 S 5 S. This assures an equal proportion of each RNA.
The rRNA operon also contains genes for some tRNAs (Fig. 22-2). The transcription product of the rRNA operon consists of a 30 S RNA; this transcript is successively cleaved and trimmed to produce the final 16 S, 23 S, and 5 S RNAs that are incorporated into the small and large ribosomal subunits. Figure 22-2 presents the scheme of maturation of the prokaryotic rRNAs.
For clarity, the incorporation of the ribosomal proteins is not shown. Ribosomal proteins combine with the rRNAs at various stages of subunit assembly: some are incorporated during transcription, others following release of the primary transcript and during processing, and still others once the mature rRNA products are formed. Certain proteins bind to the rRNAs only transiently and are not found in the fully assembled subunits.
Multiple copies of the rRNA genes occur in the genomes of prokaryotic (and eukaryotic) cells (Table 22-3); this is known as reiteration. In E. coli the number of rRNA genes is estimated to be between 5 and 10 and accounts for about 0.4% of the cell’s total DNA. The primary structures of the three prokaryotic rRNAs have been extensively studied. 5 S RNA was identified in 1963 and, being the smallest of the three rRNAs (about 120 nucleotides), was sequenced first (in 1967).
Analyses of the nucleotide sequence of the E. coli 16 S RNA (1542 nucleotides) and 23 S RNA (2904 nucleotides) have recently been completed and the secondary structure of the 16 S RNA is shown in Figure 22-3. Methylation of certain bases in the sequence of 16 S RNA (and also in the sequence of 23 S RNA) occurs while transcription is taking place. No methylation of 5 S RNA nucleotides occurs.
Unlike 5 S RNA in which duplication of certain sequences occurs, no repeated sequences are found in 16 S and 23 S RNA. The rRNAs contain a number of double-helical regions that are stabilized by conventional, complementary base pairing. In E. coli 16 S rRNA, about half of all nucleotides present are involved in base pairing. Several palindromes (base sequences reading the same from either the 5′ or 3′ ends) exist in 16 S RNA, and these may play a role in restricting the formation of the double-helical regions.
ADVERTISEMENTS:
In 16 S RNA, a seven-nucleotide segment of the chain at the 3′ end is believed to interact with mRNA, leading to its binding during the initiation of translation. The 5 S and 23 S RNAs interact with one another in the large subunit, and both appear to be involved in aminoacyl-tRNA and peptidyl-tRNA binding during polypeptide chain elongation. Because the 16 S RNA as well as several proteins of the small subunit interact with 23 S RNA, the latter RNA may also have a role in subunit association.
Ribosomal RNA transcripts are not translated into proteins (i.e., rRNAs cannot serve as messengers), however, ribosomal proteins are the products of a typical transcription-translation process. Protein Content Nomura, Kurland, and others have established that the small prokaryotic ribosomal subunit contains 21 different protein molecules; these are identified as S1, S2, S3 . . . S21. The large subunit contains 34 proteins (L1, L2, L3 . . . L34) but only 31 are different, that is, four copies of one of the proteins are present.
The genes for the 52 different ribosomal proteins together with those for the three RNAs constitute about 5% of the genome of the bacterial cell. All the ribosomal proteins have been isolated and characterized, and nearly all have been fully sequenced. The small subunit proteins range in molecular weight from 10,900 to 65,000 and the large subunit proteins vary in molecular weight from 9600 to 31,500 (Table 22-4). Most of the ribosomal proteins are basic in nature, being rich in basic amino acids and having isoelectric points around pH 10 or higher.
An exhaustive analysis of the primary structures of prokaryotic ribosomal proteins done in order to evaluate their degree of homology indicates that these proteins did not have a common evolutionary ancestor. Homologies among them do not occur more often than would be expected on a random basis.
tRNA-Protein Interaction:
Lake, Nomura, Wittmann, Traut, Stoffler, Kurland, and others have studied the relationships between the three rRNAs and the ribosomal proteins and have shown that about 30 proteins bind specifically and directly to the rRNAs; these are the primary binding proteins.
Those proteins that do not bind directly to rRNA (i.e., the secondary binding proteins) interact with the primary binding proteins in the assembled ribosome. Figure 22-4 shows that approximate regions of 16 S RNA with which the small subunit proteins associate. It is believed that the backbone of the 16 S RNA polynucleotide winds its way among the proteins, with interactions occurring between hairpin turns of the RNA and surface residues of the protein molecules.
Assembly of Prokaryotic Ribosomes:
Because all of the proteins and RNAs of the prokaryotic ribosome subunits may be isolated, it is possible through recombination studies to examine the assembly process. Nomura and others have shown that the assembly of individual subunits and their association to form functional ribosomes (i.e., ribosomes capable of translating mRNA into protein) occur spontaneously in vitro when all the individual rRNAs and protein components are available.
Thus the ribosome is capable of self-assembly, and this is believed to be the mechanism in situ. The assembly is promoted by the unique and complementary structures of the ribosomal protein and RNA molecules and proceeds through the formation of hydrogen bonds and hydrophobic interactions. There is order to the assembly in that certain proteins combine with the rRNAs prior to the addition of others. Cooperativity also exists, because addition of certain proteins to the growing subunit facilitates addition and binding of others.
ADVERTISEMENTS:
No self-assembly takes place when L proteins are added to 16 S RNA or when S proteins are added to 5 S and 23 S RNA. However, it is interesting to note that RNA from the 30 S subunit of one prokaryotic species will combine with the S proteins of another prokaryote to form functional subunits. The same is true for 50 S subunit proteins and RNAs from different prokaryotes.
Assembly of hybrid subunits and formation of functional monomers from these occur in spite of the fact that ribosomal proteins and RNAs from different prokaryotes have different primary structures. It is clear that their secondary and tertiary structures, which are very similar, are more important in guiding rRNA-protein interactions. Although some proteins from yeast, reticulocyte, and rat liver cell ribosomes can be replaced by E. coli ribo- somal proteins, hybrid monomers formed from these prokaryotic-eukaryotic subunits will not function in protein synthesis.
Model of the Prokaryotic Ribosome:
Based on the available electron-microscopic data, results of small-angle X-ray analysis, and, of course, chemical studies, and several proposals can be made about the structure of the ribosome monomer and its sub- units. The 30 S subunit approximates a prolate ellipsoid of revolution (Fig. 22-5a).
A transverse partition or groove encircles the long axis of the subunit, dividing it into segments of one-third (i.e., the head) and two-thirds (i.e., the base). A small protuberance called a platform extends from the base. The 50 S subunit is somewhat more spherical and possesses a flattened region on one surface (Fig. 22-5a). Extending from the main body of the large subunit are a stalk and central protuberance. Association of the subunits to form the 70 S monomer is depicted in Figure 22-5b.
There is considerable morphological and biochemical evidence supporting the idea that the small tunnel formed between the two subunits upon their association (Fig. 22-5b) is the site of mRNA and aminoacyl- tRNA binding during protein synthesis (Fig. 22-5c).
For example,
(1) In many electron photomicVographs of polyribosomes, the thin mRNA’ strand seems to “disappear” into the ribosomes;
(2) In vitro experiments have shown that when the synthetic messenger polyU is associated with the 70 S monomer, the polynucleotide is protected from ribonuclease attack over a length of about 70 to 120 nucleotides; and
(3) Transfer RNA is protected from cleavage by nuclease when associated with the ribosome. The observation that small nascent (i.e., growing) polypeptides are protected from proteolysis suggests that a stretch of the polypeptide chain may be enclosed within a second tunnel formed in the large subunit (Fig. 22-5c).
Genes for Ribosomal RNA and Protein:
The genome of E. coli and other prokaryotes consists of a single, long, circular DNA molecule supercoiled and packed into the “nuclear” region of the cell. The E. coli chromosome is about 1100 nm long and appears to contain at least three separate regions coding for rRNA. Each region contains closely linked 5 S, 23 S, and 16 S rDNA genes.
ADVERTISEMENTS:
Because some 5 to 10 copies of each gene occur in the genome, more than one copy of each gene is likely present in each rDNA region. Genes coding for ribosomal proteins are present in at least two separate regions of the E. coli chromosome. The same regions also appear to contain genes for RNA polymerase, some transfer RNAs, and the elongation factors required for protein biosynthesis.
Lake, Nomura, and others have employed the techniques of immune electron microscopy and neutron diffraction to map the surface of the ribosomal sub- units and identify the positions of the ribosomal proteins (Fig. 22-6).
Eukaryotic Ribosomes:
The cytoplasmic ribosomes of eukaryotic cells differ from those of prokaryotes in both size and chemical composition (Table 22-2, Fig. 22-1). The monomer has a sedimentation coefficient of 80 S and is formed from 40 S and 60 S subunits. In addition, ribosomes occur in two states in the cytoplasm.
They may be associated with cellular membranes such as those of the endoplasmic reticulum (i.e., “attached” ribosomes) and engaged in the synthesis of secretory, lysosomal, or membrane proteins or they may be freely distributed in the cytosol. The functional differences between attached and free ribosomes will be pursued later, but let us turn first to a consideration of the chemical and morphological characteristics of eukaryotic ribosomes.
RNA Content:
The small subunit of the eukaryotic ribosome contains one molecule of 18 S RNA (MW 0.7 x 106) and large subunit contains 28 S (MW 1.7 x 106), 5 S (MW 3.2×104), and 5.8 S (MW 5 x 1(H) RNAs. Hence, in addition to molecular weight or size differences, a major distinction between the RNA complements of prokaryotic and eukaryotic ribosomes is the presence of an additional molecule of RNA (i.e., 5.8 S RNA) in the large subunit of eukaryotes.
18 S, 5.8 S, and 28 S rRNAs are the transcription products of closely linked genes in the chromosomes of the nucleolar organizing region (NOR) of the cell nucleus. Considerable redundancy exists as hundreds, perhaps even thousands, of copies of these rRNA genes are believed to be present (see Table 22-3). The genes for 5 S RNA are not present in the NOR but occur elsewhere in the nucleus. Consequently, unlike prokaryotes in which the 5 S RNA genes are linked to the genes for other rRNAs, the 5 S RNA genes of eukaryotes occur separately in the nucleus.
Figure 22-7 depicts the transcription and post- transcriptional processing of eukaryotic rRNAs. As in prokaryotes, transcription of DNA is mediated by the enzyme RNA polymerase; however, in eukaryotes there are three forms of this enzyme, each producing different transcription products.
The RNA polymerases of, prokaryotes and eukaryotes are compared in Table 22-5. The high-molecular-weight primary transcript, 45 S RNA, contains the precursors of 18 S, 5.8 S, and 28 S rRNAs. About half of the 45 S RNA molecule is represented by spacer sequences at the 5′ end of the transcript and between the presumptive rRNAs. The spacers are removed during processing.
As shown in Figure 22-7, the 5.8 S RNA produced during processing becomes hydrogen bonded to the 28 S RNA and the complex is incorporated into the 60 S subunit. Not shown in Figure 22-7 but discussed later is the incorporation of the ribosomal proteins. It should be noted that 5 S RNA is a primary transcription product and is not the product of posttranscriptional trimming.
Protein Content:
Various studies have established that the small subunits of eukaryotic ribosomes contain about 33 proteins (S1, S2, S3, etc.) and the large subunits 45 proteins (L1, L2, L3, etc.). The proteins of eukaryotic ribosomes are not only more numerous but also have greater average molecular weights than those of prokaryotic ribosomes (Table 22-4). From a chemical standpoint, eukaryotic ribosomal proteins have similar general properties as those in prokaryotes (e.g., rich in basic amino acids, high isoelectric point, etc.). Certain eukaryotic and prokaryotic ribosomal proteins reveal homologous regions, and these homologous proteins appear also to be functionally similar.
Nucleolar Organizing Region:
Eukaryotic cells contain several hundred copies of the genes encoding rRNA. These genes are arranged in a tandem fashion on one or more chromosomes of the nucleus. The DNA sequences between successive rDNA regions are not transcribed and represent spacer DNA. The rRNA genes and the spacer segments are usually looped off the main axis of the chromosome and are referred to as the nucleolar organizing region. It is here that most of the rRNA is synthesized. The NOR coalesces with nuclear proteins and forms visible bodies known as nucleoli.
Most eukaryotic cells contain one or a few nucleoli, but certain egg cells are a striking exception. The oocytes of amphibians (e.g., the clawed toad, Xenopus laevis) are extremely large cells and are engaged in the synthesis of especially large quantities of cellular protein. These cells produce large numbers of ribosomes in order to provide the means to sustain such quantitative protein synthesis.
Accordingly, it is not unusual to find hundreds or thousands of nucleoli (and NORs) in the nuclei of these cells. Such large numbers of nucleoli are the result of gene amplification—the differential replication of the rRNA genes of the genome. The ribosomes produced in the oocyte serve its needs for protein synthesis from the period prior to fertilization through the first few weeks of embryonic development.
By gently dispersing nuclear fractions isolated from oocytes of the amphibian Triturus viridescens and “spreading” the material on grids, 0. L. Miller and B. R. Beatty in 1969 were able to obtain photomicrographs showing transcription in progress. Since then, a number of other investigators have extended the same approach to mammalian oocytes and to spermatocytes and embryo cells from various organisms. The visualization of transcriptional activity is achieved most easily with spread nucleoli because of the high degree of rDNA gene amplification (Fig. 22-8).
The tandem rDNA genes are serially transcribed by RNA polymerases to produce 45 S rRNA. The rRNA (apparently complexed with protein) appears as a series of fibrils of varying length extending radially from an axial, linear DNA fiber (Fig. 22-9).
This feather- shaped or “Christmas tree” regions are called matrix units. The spaces between successive matrix units are nontranscribed spacer DNA segments. The ribonucleoprotein (RNP) fibrils are seen to be in various stages of completion. The short fibrils near the tip of each feather are RNA molecules whose synthesis has only just begun and the longest fibrils represent RNA molecules whose synthesis is almost complete. Hence, the direction of rDNA transcription is apparent in the photomicrograph. In high magnification views (Fig. 22-9), even the RNA polymerase enzyme molecules carrying out the transcription of the DNA are visible along the axial DNA fiber.
Success in visualizing transcription has not been restricted to nucleolar genes. Almost identical results have been obtained with non-nucleolar chromatin. Here, however, the RNA transcripts represent messenger RNA.
Dispersed and spread nuclear fractions contain non- transcribing DNA as well as matrix units (Fig. 22-9). The succession of nucleosomes reveals itself as a series of beadlike structures along the DNA fiber. Regions in which DNA is undergoing replication (called replicons) can also be seen (Fig. 22-10). S. L. McKnight and O. L. Miller have shown that DNA of homologous “daughter” fibers of the replicon also occurs as chains of nucleosomes, suggesting that replication may not require dissociation of nucleosomes or that nucleosomes are almost immediately reformed. Transcriptional activity can be identified within a replicon (Fig. 22-11), indicating that the newly synthesized DNA is almost immediately available for transcription. The growing RNA fibrils are seen in homologous regions of both chromatid arms of the replicon.
Assembly of Eukaryotic Ribosomes:
The assembly of eukaryotic ribosomes is more complex than that of prokaryotic ribosomes. The principal stages of the process are outlined in Figure 22-12. Transcribed 45 S RNA combines with proteins in the nucleolus to form ribonucleoprotein complexes.
However, not all the protein molecules of the complex become a part of the completed ribosomal subunit. Instead, certain proteins are released as RNA processing ensues; these “nucleolar proteins” return to a nucleolar pool and are reutilized. Those proteins that are retained during processing and become part of the completed subunits are, of course, legitimately called “ribosomal proteins.”
In the same sense, not all of the RNA of the complex becomes part of the ribsomal subunits, for the spacer is also processed out. (It should be noted that the spacer RNA is produced by transcription of rDNA and not the spacer DNA between genes.) Processing produces three classes of fragments. One class contains spacer RNA and nucleolar proteins.
The spacer RNA is hydrolyzed and the free nucleolar proteins return to the pool. A second class of RNP fragments contains a complex of 18 S RNA and certain ribosomal proteins that give rise to 40 S subunits. The third class of RNP fragments, which contains 38 S and 5.8 S RNA and ribosomal proteins, combines with 5 S RNA transcribed from extranucleolar rRNA genes and gives rise to 60 S subunits.
Like the genes for 45 S RNA, the extranucleolar 5 S RNA genes occur in multiple tandem copies. Among the various proteins synthesized in the cytoplasm using ribosome subunits derived from the nucleus are the ribosomal proteins themselves. These apparently reenter the nucleus for incorporation into new RNP complexes.
Model of the Eukaryotic Cytoplasmic Ribosome:
In spite of the differences in overall sizes (as manifested in the greater molecular weights, sedimentation constants, sizes, and numbers of RNAs and proteins), the cytoplasmic ribosomes of eukaryotes are remarkably similar in morphology to those of prokaryotes. As in 20 S subunits of prokaryote ribosomes, the 40 S eukaryote subunit is divided into head and base segments by a transverse groove (Fig. 22-13).
The 60 S subunit is generally rounder in shape than the small subunit, although one side is flattened; this is the side that becomes confluent with the small sub- unit during the formation of the monomer (Fig. 22- 13). The synthesis of proteins that are to be dispatched into the intracisternal space of the endoplasmic reticulum (ER) is carried out by ribosomes that attach to the membranes of the ER. As seen in Figure 22-13, attachment occurs via the large subunit.
Free and Attached Ribosomes:
The cytoplasmic ribosomes of eukaryotic cells can be divided into two classes: (1) attached ribosomes and (2) free ribosomes (Fig. 22-14). Attached ribosomes are ribosomes associated with intracellular membranes, primarily the endoplasmic reticulum, whereas free ribosomes are distributed through the hyaloplasm or cytosol. Attached and free ribosomes are chemically the same.
Although all animal and plant cells contain both attached and free ribosomes, the proportion of each varies from one tissue to another and can be caused to shift within a tissue in response to the administration of certain substances, notably hormones and growth factors. Membranes of the endoplasmic reticulum that contain attached ribosomes constitute what is called “rough” ER (or RER) and membranes that are devoid of ribosomes are called “smooth” ER (SER).
For many years, there has been considerable controversy about the functions of attached and free ribosomes. The currently accepted view suggests that proteins destined to be secreted from the cell or to be incorporated into such intracellular structures as lysosomes (which may or may not release their contents to the cell exterior) are synthesized on attached ribosomes.
For example, many of the proteins circulating in the blood plasma are derived via secretion by the liver, and these plasma proteins are known to be synthesized exclusively by the attached ribosomes of the liver cells. Most proteins destined to become constituents of the ER membranes or the plasma membranes are also synthesized by attached ribosomes.
Most, but not all, proteins destined for use in the cytosol are synthesized by free ribosomes. Exceptions include certain hormones like thyroglobulin, which is secreted by the thyroid gland and is synthesized by free ribosomes. Milk proteins produced by mammary gland cells are also synthesized by free ribosomes.