ADVERTISEMENTS:
The exact mechanism that couples the energy of electron transfer to the phosphorylation of ADP remains somewhat speculative.
However, at the present time the chemiosmotic-coupling hypothesis-proposed by P. Mitchell is a widely supported model.
Since its initial proposal in 1961, the hypothesis has undergone modification by Mitchell and by others.
ADVERTISEMENTS:
For his contributions to the development of this model, Mitchell received the Nobel Prize in 1978. According to Mitchell, a gradient of hydrogen ions is created across the inner membrane as the electron carriers pump H+ from the matrix into the inter-membrane area of intact mitochondria or into the sub-mitochondrial vesicles.
This process creates an electrochemical gradient (designated ∆μH+) across the membrane consisting of a negative-inside membrane potential (∆ψ) and an acid- outside gradient of H+ (∆pH). An intact vesicular membrane is essential for the establishment of this electrochemical gradient. The gradient, in turn, provides the energy to drive the synthesis of ATP.
It is proposed that the electron carriers involved in electron transport are not randomly arranged in or on the inner membrane but are spatially oriented in a specific manner. As electrons are transferred through the sequence of membrane carriers, there is an accompanying expulsion of H+ from the matrix. This causes the matrix to become especially alkaline (low H+ and high OH– content).
ADVERTISEMENTS:
In Figure 16-28, these events are depicted with the electron transport intermediates arranged in order in the membrane. The vectorial flow of protons from matrix to inter-membrane space shown in Figure 16-28 creates the electrochemical gradient that is the source of energy for phosphorylation.
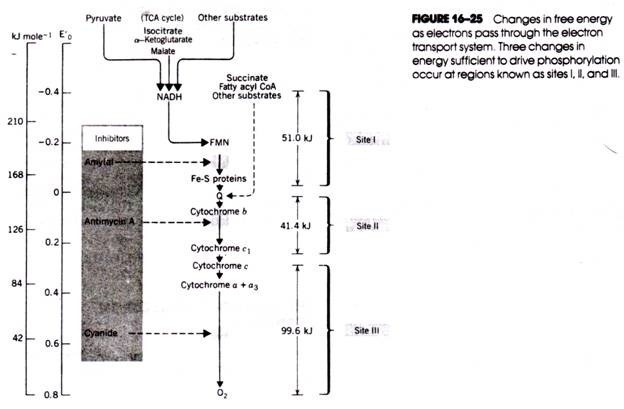
As oxidative phosphorylation proceeds, the alkaline sink within the matrix and the external acid pool are both neutralized. Continued electron transport is necessary to maintain the pH gradient that drives phosphorylation. For many years, it was believed that only two H+ were expelled from the matrix at each of the three electron transfer stages coupled to phosphorylation (i.e., sites I, II, and III in Fig. 16- 25). Now there is evidence that 12 H+ are transferred across the membrane (see Fig. 16-29).
There is good evidence, both direct and indirect, to support the chemiosmotic-coupling hypothesis. Phosphorylation of ADP also occurs in chloroplasts and can be explained by a similar chemiosmotic-coupling mechanism that creates a proton gradient across the organelle’s thylakoid membranes. Indeed, A. Jagendorf has shown that a burst of ADP phosphorylation occurs when chloroplasts isolated from cells at neutral pH are placed in a buffer solution atlowpH.
Whereas electron transport is accompanied by the outward transport of H ATP synthesis is linked to the inward movement of H+. Because ATP synthesis occurs on the matrix surface of the inner membrane, ADP and inorganic phosphate must be transported from outside the mitochondrion across the membrane and into the matrix.
The inward transport of P; and ADP and the outward transport of ATP are driven by the electrochemical gradient (see Fig. 16-29). One H+ reenters the matrix along the pH gradient during the inward movement of inorganic phosphate, and at least two more H+ (perhaps as many as three more) reenter the matrix for each molecule of ATP synthesized (Fig. 16-30).
Although the chemiosmotic-coupling model is widely accepted, the precise mechanism resulting in ATP synthesis remains uncertain. Also unexplained is how the protons are transported chemically. The similarities in structure, properties, and function of ATP synthetases and hydrogen ion pumps that have recently been isolated from various animal, plant, and microbial cells suggest that some answers will be forthcoming.