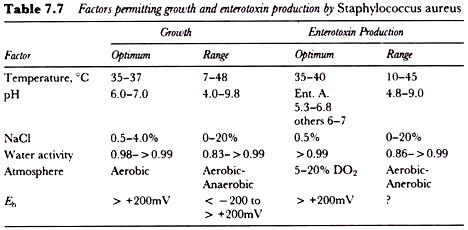
ADVERTISEMENTS:
(1) Microbial Cell Process:
Theoretically the following criteria were assumed suitable basis for scale up of bioreactors.
A. Constant power input per unit volume (P/V = constant).
ADVERTISEMENTS:
B. Constant KLa
C. Constant mixing quality
D. Constant momentum factor (MF = ND. NWL (D – W)) = constant)
E. Similar drop size distribution (ds = constant)
ADVERTISEMENTS:
F. Constant impeller tip speed (π NDi = constant)
G. Constant mixing rate number = (N/K)(Di/Dt)α constant
Short accounts of scaling up of bioreactors of fermenters on the basis of these criteria are discussed below;
1. Constant power input per unit volume:
For scaling up based on these criteria it is necessary to consider whether it is gassed or non-gassed system. From the work of Rushton and his associates, for geometrically similar, fully baffled vessels with turbulent conditions, it may be noted that if scale up should be based on maintaining a constant power input per unit volume considering no gassing in the system one may have the following.
(i) Non-gassed System:
When there is no gassing in the system then based on constant P/V has the power number, Np as
It was also apparent that in an oil drop dispersion system ds could serve as important criteria for scale up. The value of “a” in equation 8.34 depends on the type of agitator used in the system. For turbine impeller a= -0.6 whereas for draft tube fermented its value ranges between -0.15 to -0.35.
6. Constant impeller tip speed:
ADVERTISEMENTS:
It was rather surprising to recognize that most of the data on impeller tip speeds collected were in the range 5-7 m sec-1 indicating the predominant importance of this parameter in scale up. Specially in antibiotic production plants constancy in tip speed (π NDi) was encountered in several cases. For scale up
In equation (8.37) N represents impeller tip speed and Di1 impeller diameter
7. Constant mixing rate number:
ADVERTISEMENTS:
In scale up of bioreactors mixing time is often used as criteria. Mixing time (tmix), defined as the period of time required for the homogeneous distribution of a small volume of pulsating material in the bulk of the liquid, was used for scale up to adjust proper mixing conditions in large vessels. However, limitation of using tmix as scale up criteria is that it increases markedly with the size of the vessel and it is difficult to maintain to constant in both vessels at reasonable power expenditure.
In mixing a fermentation broth two vital questions to be answered are:
(1) What size of motor is needed for a given impeller rotation speed? and
(2) how long the broth must be mixed to achieve a required degree of homogeneity?
ADVERTISEMENTS:
From the present knowledge the first question can be answered using Np – NRe relations of Rushton and Bates et al. The answer of the second question has been provided8 by introducing a new dimensionless term mixing rate number [(N/K) (D/Dt)a], NMR in the NP – NRe profile to characterize the rate of approach to uniformity in mixing vessels. This profiles as shown in Fig. 8.1 a and b indicate that for large impeller Reynolds number (NRe > 104) the following correlations will be applicable depending on the type of mixer used.
For turbine impeller mixer
(2) Scale Up of Filamentous Cell Fermentation:
ADVERTISEMENTS:
Aerobic fermentative bioprocessing using filamentous microorganisms cover a wide range of industrially important bio-product formation systems. Various Streptomyces sp. antibiotic fermentations fall in this group. Such bioprocessing exhibit viscous and non Newtonian behavior following Power law fluid characters.
These processes are typical and present considerable problems in mixing and mass transfer for scale up purpose. Thus, agitator power number (NP) and impeller diameter (Di) are important case factors towards implications of Power law fluid on scale up along with liquid volume (VL) and (HL /Dt) ratio.
Case 1:
Agitator power (Pt) number influence
a. Provided with same geometry, vs, (Pt/Vt), VL and Di manipulation of volumetric mass transfer equations show
(3) Mammalian Cell Processing Scale Up:
In scaling up bioreactors of mammalian cell bioprocess most of the correlations are based on laboratory models alone and still awaits confirmation of their utility in industrial production concern. For mammalian cell bioprocessing current industrial scales typically involve bulk liquid volume of 25/ per day per bioreactor for continuous or semi-continuous systems.
In batch processing this bioreactor volume ranges between 500-5000 I capacities, operating over 1-3 weeks. There are no established scales up criteria for scaling up mammalian cell culture process. Cell culture engineers therefore, still largely depend on concepts and scale up procedures developed for chemical or microbial/biochemical processes to begin with.
However, an engineering body of experience and data will increasingly enable more specific design engineering and scale up procedures for mammalian cell culture bioprocesses in which kinetics is likely to be influenced by many factors and associated with many problems and limitations.
In scaling up procedures specific to animal cell culture, effect of scale on oxygen mass transfer through micro-porous membranes like silicone tubes for supplying bubble free oxygenation has been investigated. In order to aerate cell cultures, the membrane is immersed in the medium and the gas mixture (e.g. air, CO2 and O2) is passed through the tube under pressure.
Depending on the difference in pressure between the gas and the medium, gas flows through the membrane tube. Bubble free aeration is achieved if the internal gas pressure does not exceed the pressure at which bubbles will form. Correlation for bubble free oxygenation through membrane tubes has been developed.
For this, novel bioreactors have been designed for cell culture engineering in large quantities of the fragile complex mammalian cells that synthesize commercially and medically important proteins such as interferon and monoclonal antibodies. The correlation for bubble free oxygen using silicone micro-porous tube for oxygen mass transfer in this novel bioreactor has been given by the following correlations.
In these correlations, Sh is Sherwood number, K is film mass transfer coefficient (m s_1), d is tube diameters, D0 is molecular diffusivity of oxygen (m2 s-1), e, f, g are specific constants, Dt is bioreactor diameter (m) and NRe is impeller Reynolds number.
The provision of an adequate oxygen supply to large volumes of mammalian cells is the most crucial barrier to scale up, especially in suspension systems. Oxygen is only sparingly solube in cell culture medium (0.2 m mole O2I-1). Oxygen demand of a culture (106 cells per ml) ranges between 0.053 m mole O2 l-1 h-1 and 0.59 m mole O2 I-1 h-1 depending on the type of cell. Scale up of mammalian cell cultures are, therefore, bound by several barriers as shown in Table 8.1.
As d, D0, NRe and Dt are given and e, f and g are determined experimentally, then equation 8.48 enables calculation of the oxygen mass transfer coefficient from impeller speed. With the assumption that gas velocity and composition within the silicone tube are constant, one may calculate the power required for this form of aeration at the large scale using the correlation given below.
In this correlation P, is the power input per unit mass of liquid (W.kg-1), V is the reactor volume (m3), D; is impeller diameter (m), p is the kinematic liquid viscosity (m2 s-1) and NP is the power number. Thus, scale up might be carried out on the basis of impeller speed to yield a constant oxygen mass transfer rate, but the power input per unit mass will vary with scale. It will need to check the power against an upper limit which will be determined by the shear sensitivity of the mammalian cells.
Lavery and Ninow in their study of the conventional power input versus mass transfer relation in sparged mammalian cell culture indicated the necessity of consideration of both the effect of head space aeration and the effect of added antifoam. This could account for the deviations from the expected values of KLa in their result.
A number of serious problems associated with the design and scale up of high density perfusion cultures of mammalian cell bioprocessing have been pointed out. For overcoming the associated problems in cultivation of animal cells and their scale up the following usage has been suggested.
1. Micro bubble technology and soluble surfactants for meeting cellular oxygen uptake rate (OUR).
2. Turbine or low shear impellers for achieving well mix cultures, such that it provides independent requirements of mixing and bubble aeration.
3. Minimization of ammonia accumulation by low glutamine supplemented media design.
4. Appropriately sized microfiltration hollow fibre cartridges which are steam sterilizable and easily cleaned after repeated uses.
(4) Plant Cell Processing Scale Up:
A typical plant cell culture manufacturing process is shown in Fig. 8.2. The great upsurge of interest in process biotechnology in the 1970’s carried plant cell culture with it. This is primarily because of centuries plants have been an important source of drugs and chemicals.
The extensive effort in development of plant cell culture is evident from the literatures on the subject. As plant cell cultures have many differences in comparison to microbes the design and scale up of suspension cultures bioreactors for plant cells also differ to a great extent. This becomes more pronounced as plant cell cultures can achieve high cell densities and viscosities. In such systems need for good mixing must have a compromise to shear sensitivity of plant cells.
Thus, in plant cell culture scale up system information is very important. Useful system information’s for scale up of plant cell culture as proposed by Goldstein are presented in Table 8.2. In addition to these quantization of entities as effected through bioprocess engineering studies at laboratory scale needs information on operational variables as listed in Table 8.3.
The first consideration in generating these information’s is the selection of a suitable bioreactor type. Continuous stirred tank bioreactor (CSTBR), air lift bioreactor (ALB), immobilized plant cell bioreactor etc. have been used in plant cell cultivations either in batch, plug flow or in continuous modes. In these three modes culture residence time is analyzed based on the relations given in Table 8.4. Assuming autocatalytic growth these relations emerge from classical catalysis.
The process parameters for scale up of plant cell cultures as listed in Table 8.5 demand low shear bioreactor for plant cell aggregates. As reported in literature plant cell cultures often form aggregates in suspension cultures in bioreactor. It can make the provision of oxygen difficult: the high aeration or mechanical agitation necessary can both cause shear damage to the cells.
Table 8.5 Process parameters for scale up of plant cell culture:
For overcoming the above difficulty and to facilitate scale up of plant cell culture bioreactor a design of low shear bioreactor has been reported. This design is stated to create separate cell growth and aeration zones. The bioreactor design is analogous to fluidized bed columns except that the cells are immobilized naturally (by aggregation).