ADVERTISEMENTS:
Read this article to learn about the various production of commercial products by the use of bio-transformation.
1. Biotransformation of Steroids:
All the steroids possess the basic structure namely cyclopentanoperhydrophenanthrene. Steroids as hormones (glucocorticoids, mineralocorticoids, androgens, estrogens) perform a wide range of functions. They are very useful therapeutically. For instance, cortisone, due to its anti-inflammatory action is used in the treatment of rheumatoid arthritis and skin diseases; derivatives of progesterone and estrogens are employed as contraceptives. Certain derivatives of cortisone (e.g. prednisolone) are more effective in their therapeutic action.
Commercial production of steroids is very important. Cortisone was chemically synthesized, and this process involved as many as 37 reactions. The cost of the so obtained product was around $200/g (in 1950). With the introduction of biotransformation reactions, the number of steps (microbial and chemical put together) was reduced to II, and cost of the product was reduced to just $1/g in 1980! The credit obviously goes to the developments in biotransformation.
ADVERTISEMENTS:
Types of reactions in biotransformation of steroids:
The microbial transformation of steroids broadly involves oxidation (introduction of hydroxyl groups, splitting of side chains, production of epoxides etc.) reduction (conversion of aldehydes or ketones to alcohols, hydration of double bonds), hydrolysis and ester formation.
Production process of steroids:
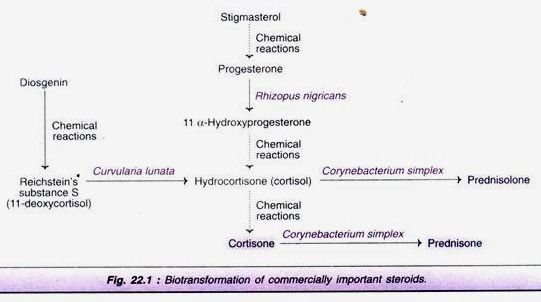
The production of steroids, entirely by biotransformation reactions is not practicable. Therefore, microbial transformation along with chemical reactions is carried out. The major steps involved in the biotransformation of steroids are depicted in Fig. 22.1. Stigma sterol extracted from soybeans or diosgenin isolated from the roots of the Mexican barbasco plant can serve as the starting material.
Stigma sterol can be chemically converted to progesterone which is subjected to biotransformation to form 11 α-hydroxyprogesterone by the microorganism, Rhizopus nigricans. Cortisol (hydrocortisone), produced from 11 α-hydroxyprogesterone by chemical reactions, undergoes microbial transformation (organism-Corynebacterium simplex) to form prednisolone.
Further, cortisone formed from Cortisol can be subjected to biotransformation by Corynebacterium simplex to produce prednisone. When diosgenin is used as the starting compound, substance S can be produced by chemical reactions which can be converted to Cortisol by biotransformation with the help of the microorganism Curvularia lunata.
Biotransformation of steroids is usually carried out by batch fermentation. Use of immobilized cells or immobilized enzymes is gaining importance in recent years. This is advantageous since the biotransformation is more efficient with high substrate concentration, short conversion time and good product recovery.
Since the steroids are not water soluble, the microbial transformation reactions have to be carried out in organic solvent (water-immiscible) system. However, the organic solvents are toxic to micro-organisms or enzymes. It is ideal to use an aqueous two phase system for biotransformation of steroids.
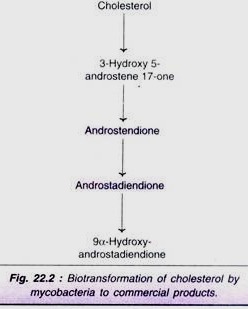
Biotransformation of cholesterol:
Certain commercially important steroids (e.g. androstendione, androstadiendione) can be produced directly from cholesterol by biotransformation (Fig. 22.2).
2. Biotransformation of Antibiotics:
Production of new antibiotics or modifications in the existing ones for more effective treatment of the diseases is always on the priority of the pharmaceutical industry. Further, antibiotics with wider antimicrobial spectrum, reduced toxicity, low allergic reactions and decreased resistance are highly advantageous. Biotransformation reactions significantly contribute for improving the pharmaceutical products.
ADVERTISEMENTS:
Direct biotransformation:
Acylation and de-acylation, phosphorylation, adenylation and hydrolysis are some of the reactions involved in the microbial transformation of antibiotics.
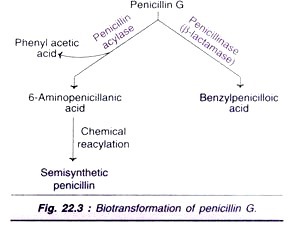
Biotransformation of penicillin G:
ADVERTISEMENTS:
Microbial transformation, in association with chemical synthesis, is routinely used for the commercial production of semisynthetic penicillin’s and cephalosporin’s. The enzymatic cleavage of penicillin by penicillin acylase into 6-amino- penicillanic acids is a very important reaction (Fig. 22.3). Penicillin G gets inactivated by its conversion to benzylpenicilloic acid by the enzyme penicillinase (β-lactamase).
ADVERTISEMENTS:
Biotransformation of narbomycin:
Hydroxylation of narbomycin to picromycin (brought out by Streptomyces sp) is another good example of microbial transformation.
ADVERTISEMENTS:
Biotransformation of macrolides:
The macrolide antibiotics on de-acylation will give less active products. These products can be used for the production of more active semisynthetic macrolides.
Indirect biotransformation:
The biosynthetic processes of antibiotics can be controlled by the addition certain inhibitors or modified substrates to the medium. In other words, the biosynthesis of antibiotics occurs in a controlled fashion in the indirect biotransformation.
Biotransformation of actinomycins:
The microorganism Streptomyces parvulus produces new actinomycins in the presence of 4-methyl- proline (proline analog) in the medium. The new antibiotics will have 4-methylproline in place of proline and these actinomycins are more efficient in their function.
ADVERTISEMENTS:
Biotransformation of ribostamycin:
In the biosynthesis of neomycin, ribostamycin is an intermediate. By employing mutant strains of Streptomyces fradiae, ribostamycin can be produced in large quantities. Several other mutant strains of microorganisms have been created by recombinant DNA technology for the production of modified antibiotics of aminoglycosides and rifamycins.
3. Biotransformation of Arachidonic Acid to Prostaglandins:
Prostaglandins (PG) have a wide spectrum of biological functions. They are important for pharmaceutical and therapeutic purposes. For instance, PGE1 serves as a contraceptive; PGG1 is used in the treatment of congenital heart failure; PGG2 for relieving labour pains.
The unsaturated fatty acid arachidonic acid is the precursor for the biosynthesis of prostaglandins. Some success has been reported in the biotransformation of arachidonic acid to PGE1, PGE2, PGF1 and PGF2 by using fungi. It is expected that in the coming years, prostaglandins with improved efficiency will be produced by bio-transformations.
4. Biotransformation for the Production of Ascorbic Acid:
Ascorbic acid (vitamin C) can be commercially produced by a combination of chemical and microbial transformation processes.
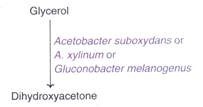
5. Biotransformation of Glycerol to Dihydroxyacetone:
ADVERTISEMENTS:
Dihydroxyacetone is used in cosmetics and suntan lotions. Certain acetic acid bacteria can convert glycerol to dihydroxyacetone through the process of biotransformation.
Good oxygen supply, temperature 26-28°C and pH 6.0 are ideal for the optimal biotransformation.
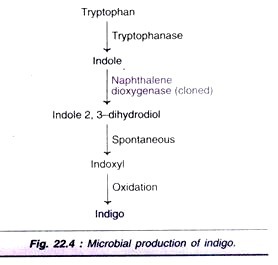
6. Biotransformation for the Production of Indigo:
Indigo can be synthesized by microbial transformation. This has been made possible by cloning a single Pseudomonas gene that encodes naphthalene di-oxygenase in the creation of E. coli. The relevant reactions of biotransformation for the production of indigo are depicted in Fig. 22.4.