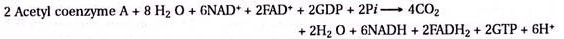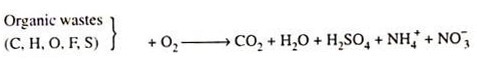ADVERTISEMENTS:
Brewery and Distillery Effluents:
Breweries and distilleries produce huge quantity of liquid effluents originated from different sections of the industrial processing.
These effluents are highly pollutants bearing in terms of BOD and COD loadings.
ADVERTISEMENTS:
1. Origin of wastes:
The malting process produces two major wastes: those arising from the steep tank after grain have been removed, and those remaining in the germinating drum after the grain malt have been removed. Brewery wastes are composed mainly of liquor pressed from the wet grain, liquor from yeast recovery, and wash water from the various sections. After the distillation of the alcohol process, a residue remains which is generally referred to as ‘distillery slops’, ‘beer slops’, or ‘still bottoms.’
There are several sources of wastes in a distillery. Of major concerns are the ‘dealcoholized’ still residue and evaporator condensate, when the stillage is evaporated. Minor wastes include re-distillation residue and equipment washes. In the manufacture of compressed yeast seed, yeast is planted in a nutrient solution and allowed to grow under aerobic conditions until maximum cell multiplication is attained. The yeast is then separated from the spent nutrient solution, compressed, and finally packaged.
The yeast plant effluent consists of:
ADVERTISEMENTS:
(1) Filter residues resulting from the preparation of the nutrient solutions,
(2) spent nutrients,
(3) wash waters,
(4) filter press effluents, and
(5) cooling and condenser waters (Tables 11.3 and 11.5).
2. Plant lay-out:
Wastes emanate from the malting, brewing and washing operations, as shown in the Fig. 11.6. In the brewing operation malt is prepared in the mash turn at elevated temperature with water containing calcium and phosphate salts. After the malting operation, the mash is discharged to a screen filter (lanter tub) from which the spent grains are removed to a hopper and trucked away. The malt liquor is then discharged to the kettle to which the hops are added.
Following heating in the kettle, the mixture is sent to a filter (hop jack) for removal of spent hops. The filtered mixture is pumped to a settling (hot wort) tank, then cooled and sent to storage (cold wort tank). The wort from storage is filtered through diatomaceous earth and passed to the fermentation tank. Yeast is added to the fermentation tank, in which a temperature of 61-70°F is maintained.
After fermentation the beer is removed to warm storage. The beer from warm storage is carbonated and filtered through diatomaceous earth and sent to cold storage. Carbonation and filtration follow and the beer are then bottled.
3. Waste water characteristics:
Malt wastes
Plant capacity : 6996 bushels of barley/day.
Suspended solids : 72 mg/l
ADVERTISEMENTS:
5-day BOD (20°C) : 390 mg/l
Water consumption : 75 gallons/bushel.
The solids are mainly organic and high in nitrogen, indicating considerable proteinous material. A major portion of the solids was in solution, as indicated by the low suspended solids content. Yeast wastes consist primarily of the spent nutrient (although only 20 per cent of the wastes by volume, they account for 75 to 80 per cent of the total BOD). They are brown, have a typical odour of yeast, and are highly hygroscopic. The solids are almost entirely dissolved and colloidal; the suspended solids content is seldom above 200 mg/l. The composition of this waste is given in Table 11.6. Characteristics are given in Table 11.7.
4. Distillery effluent in biogas generation:
(i) General Considerations:
ADVERTISEMENTS:
India has more than 160 molasses based distilleries. About 900 million liters of distillery effluent are generated annually by them. It is one of the most polluting and environmentally unacceptable organic liquid wastes which cause health hazard to the people in the surrounding locality.
The major sources of liquid waste water for molasses based distilleries are:
(a) Process waste stream like spent wash and digester sludge, and
(b) Non- process waste stream like cooling water, boiler blow down water etc.
Government stringent regulations as well as mass health considerations have made it a compulsion to treat this hazardous effluent using efficient process technology such as anaerobic digestion for production of biogas and abating pollution. Many biogas installations from distillery waste based on Indian Technology are now in operation in India.
A number of technologies from various countries such as U.K., USA, France, Belgium, Thailand, Canada, Switzerland and Italy have established collaboration with various Indian distilleries for biogas generation from their effluent (Tables 11.8 and 11.9). Also, in about three cases the plants are yet to be commissioned and some are in the process of being commissioned. Table 11.8 gives a list of biogas installations from distillery waste based on Indian technologies and foreign collaboration technologies on the basis of available data.
Table 11.10 provides comparative results claimed by the various technology suppliers. Some of them have claimed biogas generation from 30-35 m3 per m3 of spent wash of methane content 70% which, seems to be on the high side. Anaerobic digestion of concentrated industrial effluents from sugar mills, distilleries, breweries, paper and pulp mills, dairies, canneries, animal breeding etc. under properly controlled/regulated conditions generates large amount of methane/biogas.
It is an ideal fuel for boilers and for generating electricity or for consumption of energy in rural India. Its need for alternate energy supplies for rural development and rural environmental protection is increasingly apparent. The present energy generating system in rural parts of developing countries like India depend largely on local resources like fire wood, straw or dung cake for burning.
The smoke of these fuels creates unhealthy conditions to villagers. In order to solve this problem to a great extent and to meet the energy demand of villager’s use of biogas as a clean fuel has been recommended. So biogas concept has drawn extensive attention and importance all over the globe including India. Typical distillery effluent raw material specification is shown in Table 11.11.
It is the volatile portion of solids that provides the food for the microorganisms in anaerobic digestion system. Actually the process involves two phases from bacteriological stand point. In the first phase anaerobic acidogenic bacteria breakdown the organic fractions of the solids generating volatile organic fatty acids in the digester. These acids next become the substrate for methane producing bacteria. Total reduction (100%) of original volatile matter in the sludge is virtually non-achievable. In practice the volatile solids (VS) in the raw distillery sludge are reduced by 40 to 70%.
ADVERTISEMENTS:
In order to make the distillery effluent treatment plants economically viable one has to keep in mind that the process should provide maximum methane formation (of course without compromising the quality or life of the particular system adopted). For obtaining maximum CH4 economically every single distillery effluent treatment process has to adopt ‘Anaerobic Digestion’ bio-technology. It can be mesophilic’ or ‘thermophilic’ depending on the temperature of performance of the digester.
From a comparative view point one can observe that out of several overseas technologies only one has adopted thermophilic anaerobic digestion technology of M/S Aquatech (Thailand) which is located at the Rampur Distillery and Chemical Co. Ltd. The biogas generation has been claimed to be 29 m3 per m3 of spent wash. The methane content is 55% and thus the calorific value is 4700 k. cal/m3.
Although there are a few advantages in choosing thermophilic anaerobic digestion but the disadvantages are many. It is learnt that the same technology used at the Ashok Organic Plant has not yet yielded very satisfactory results. Indian climatic conditions are such that ‘mesophilic’ digestion is very well suited in most parts of the country.
Moreover the ‘mesophilic’ temperature range (35-40°C) is more conducive and operationally easier to methane bacteria. Not only that since the source of the distillery effluent contains lot of important materials suitable as a bio-fertilizer many investigators have tried to look into the possibility of using the sludge of the biogas disaster as a source of bio-manure as well as animal feed. Necessity of having a thorough study on the details of the anaerobic digestion biotechnology is therefore obvious.
(ii) Theoretical Considerations:
A. Molasses resources and distillery:
Molasses is the syrup that is left after the recovery of crystalline sugar from the concentrated juice of sugar cane. Depending on processing of sugar cane juice two kinds of molasses have been reported in the literature. They are ‘Blackstrap molasses’ and ‘High test molasses.’ Blackstrap molasses has been used principally in industrial alcohol production in distilleries. It usually contains 48 to 55% of sugars mainly sucrose.
However, ‘High test molasses’ has also been used for ethanol manufacture. It is an evaporated juice but most of it remains in inverted sugar form as a result of acid hydrolysis. This type of molasses is usually high in sugar content. Occasionally it may contain as high as 78 per cent sugar. In India ‘blackstrap molasses’ has been widely used for industrial alcohol production.
The industrial process adopted by the distilleries consists of fermenting the diluted molasses with yeast and distilling the alcohol produced in distillation columns by passing a counter current of steam while allowing the fermented mash to come down the tall column. Only about 10 per cent of the molasses is utilized in the process and the major remaining liquid part comes out as effluent in the process.
This liquid effluent consists of spent wash and yeast waste sludge and floor washings. Yeast waste sludge is separated out and dried and used as animal feed. After removal of yeast the liquid effluent is called distillery effluent and has been used as substrate for long time in anaerobic digester for its treatment and disposal.
B. Basic biogas technology concept:
Biogas production from available liquid waste resources from distillery effluent through anaerobic digestion for bio-methanation is an era old concept. An idea of daily availability of distillery effluent has been given in literature. As this effluent discharged by an alcohol/distillery industry is environmentally most polluting containing unacceptable organic waste in terms of COD (approx. 80,000 to 1, 00,000 ppm) and BOD (approx. 40,000 to 55,000 ppm) loading its proper disposal retrieving benefit to maximum extent is essential.
Anaerobic digestion for management and disposal of distillery effluent both in terms of utilization and protection of environmental pollution has been critically analysed by engineers taking into consideration the general properties of distillery effluent as given in the Table 11.12. Basic biogas technology comprises therefore, the bioconversion of carbonaceous material into volatile organic acids by acid forming bacteria in absence of air, subsequently bioconversion of volatile organic acids by methane bacteria in absence of air into methane.
C. Thermodynamic basis of quantitative analysis:
As can be seen from Table. 11.12 that the major component in distillery effluent is carbonaceous organic matter responsible for anaerobic bio-methanation. The stoichiometry of hydrolytic methanization of carbonaceous component has been preliminarily based on a very simple chemical equation.
However, in this simple chemical stoichiometry chemically bound nitrogen has not been considered. But nitrogen negatively affects the course of anaerobic digestion. As sometimes the content of bound nitrogen in distillery effluent may be appreciable and variable, its rise brings about a drop in the methane yield coefficient Y (CH4/s). In that case the above referred simple chemical balance need to be modified by the nitrogen and other negatively affecting elements like sulfur present.
The overall hydrolytic energy retrieval stoichiometry needs to be considered accordingly. From the knowledge of electron participation in anaerobic digestion of distillery effluent and considering the general formula of distillery effluent substrate containing nitrogen and sulfur, it is theoretically possible to measure degree of bio-reducibility of this substrate. It implies that due to the presence of elements like N, S etc. as acceptors of electrons in anaerobic digestion redox reaction has a negative effect.
Accordingly higher levels of nitrogen sulfur and other similar elements which may act as electron donors or acceptors in anaerobic digestion influence on bio-methanation. In most developing countries like India where sugar based industries produce large amounts of molasses it will be highly desirable to recycle the molasses for bio-methanation through anaerobic digestion to replace its coal/furnace oil requirement as well as to prevent pollution of the locality.
On the basis of theoretical and investigational information’s various basic biotechnological process engineering designs have been developed in many countries. Comparative design figures of them are given in Table 11.10.
In order to determine these basic biotechnological parameters of anaerobic digestion, elemental analysis of the resource substrate is very essential. It yields the empirical substrate formula referred to 1C atom and values of substrates reducibility degree (vs) and yield coefficient (Ys). These values then aid in determining the Y(CH4/s) theoretical, COD theoretical and per cent methane.
Also, the content of vs, yield coefficient, Yexp and COD are determined by experiments. If the substrate composition is unknown the value of Ys for a given biomass can be determined from the experimental COD and vs from the content of CH4 in the biogas. Measurement of these quantities during the course of digestion makes it possible to characterize semi-quantitatively the course of individual phases of the digestion process.
Using Yexp and Ytheo values it is possible to calculate the actual biotechnological efficiency (ɳ from the values of Ys determinedes % CH4 in the biogas and from vs assessed by means of COD value, the theoretical amount of heat, Q yields by combustion of CH4 from anaerobic digestion can be determined. From the known calorific value of coal it will now be possible to determine the coal/furnace equivalent of the heat obtainable from biogas. Thus the amount of coal that can be replaced by the generated biogas may be calculated.
The engineering analysis of mass and energy balance made it therefore, possible to evaluate the efficiency of different biotechnology process devices for distillery effluent digestion in biogas production.
D. Kinetic considerations:
Based on reaction rates of distillery effluent it is necessary to analyse the kinetics of anaerobic digestion. It is helpful to know the key factors affecting efficiency and stability of digestion in which solids residence time is important.
Importance of residence time:
One of the key factors for bacteria growth is residence time. To ensure efficient bioconversion of organic matter to methane and carbon dioxide, the population of bacteria in the digester must be of sufficient quality and concentration. Also they must have adequate active residence time to allow substrate metabolism and to prevent depletion of bacteria.
For the designer and operator this means providing sufficient reactor volume for a given operating condition which directly affects technology cost. The use of residence time specifically biological solids residence time (SRT), as the most important design factor is a concept that provides information on how changes in operating conditions affect performance of the technology and its reliability.
Operationally SRT has been defined as the mass of solids contained in the digester divided by the mass of solids discharged from the system per day. Depending on the operational performance efficiency various technologies have different residence times for distillery effluent. The relationship between volatile solids loading rate (VSLR) and hydraulic retention time (HRT) is important.
This relationship is dependent on influent COD and/or BOD concentration. SRT is now recognized as the most important kinetic parameter for anaerobic digester design and operation because it more accurately defines the relationship between the bacterial system and digester operating conditions as evidenced by several reports. Also, at a suitable SRT the required HRT can be very low.
Thus an efficient anaerobic digestion (AD) would involve relatively small and very economic digester. It is not desirable to design and operate anaerobic digester near maximum specific growth rate for any extended period of time. Engineers should select the proper design SRT to accomplish a given treatment efficiency with more reliability and large values of SRT.
In suspended growth digesters a large digester volume is required or else provisions for biomass recycle would be needed. In that case SRT is controlled by purposely removing solids. Process designs such as up-flow anaerobic sludge blanket process and anaerobic baffled digester all inherently provide high values of SRT at low HRT.
E. Rate limiting step:
A simplified schematic representation of the biphasic distillery effluent bioconversion reaction phases is shown in the figure Fig. 11.7.
In phase I reaction kinetics organic materials of distillery effluent are broken down to organic acids, mainly acetic, propionic and butyric acids by saprophytic acidogenic becteria and their enzymes. The principal acidogenic microbial flora consist of Acetobacter and Propionibacterium sp. Nearly 3000 – 5000 ppm of volatile acids are produced at this reaction phase. Kinetically in Phase II bioconversion of acids mainly to methane and carbon dioxide takes place by methanogenic bacteria principal flora being Methanobacterium sp., Clostridium sp. and Desulfovibrio sp.
Among these biphasic reactions, the second phase becomes rate limiting in anaerobic digestion of distillery effluent. However, although it is depending on the nature of organic substrate the rate limiting step may change for different effluent substrates.
For example, hydrolysis of complex organics becomes rate limiting during anaerobic digestion of waste where activated sludge kinetic illustration of above biphasic reactions have been described by simple kinetic breakdown of fatty acids using standard Monod’s expression. Many other factors like temperature, pH, mixing, toxicity etc. have been found to exert influence on the digestion kinetics.
(iii) Other Factors Affecting System Design and Performance:
The usefulness of kinetic considerations has been demonstrated through the relationships of the effect of SRT. Temperature, mixing, toxicity and a few other characteristics on anaerobic digestion efficiency. Reduction of BOD, COD and VS in anaerobic digestion greatly depends on the bacteria grown during digestion process. McCarty accounted the contribution of grown bacteria to reduce organic loading and noticed larger values of SRT minimized the concentration of produced bacteria in the effluent.
A. Effect of temperature:
It has been often noticed that thermophilic methanation process is less stable than a mesophilic one. In thermophilic processing temperature regime (50°C – 60°C) irregularities in loading rates did cause severe damage to thermophilic digestion. It has been reported that when the loading rate in mesophilic (35°C – 40°C) and thermophilic digesters was doubled by the reduction of HRT half gas production increased immediately by a factor of two.
It has also been reported that when temperature was lowered or increased for short (2hrs.) or long (18 hrs. or 4 day) periods the changes of daily biogas production was not very significant. Report is also available that a decrease in the digestion temperature by 3°C for 4 days or by 10°C for only 2 hrs. had no or little transient effect on the biogas production of mesophilic and thermophilic digestion system.
Lowering digester temperature to 18°C for 8 hrs however could show reduction in gas productivity by 50% in mesophilic and thermophilic digesters. The sensitivity of methanogenic bacteria to alterations in temperature, compared with other microorganisms in the digester is well known. The anaerobic digestion process has been examined to be unsuitable for dilute effluents at a low temperature.
The efficiency of conversion of carbonaceous substrate, cell growth being dependent on temperature, the maximum bioconversion product yield is expected to occur at a temperature near to maximum specific growth. So majority of distillery industries which generate significant quantities of waste heat as a component of their effluent system have utilized to maintain mesophilic temperature in their process design taking the advantage of the waste energy.
B. Effect of gas recycling and mixing:
In several units of distillery effluent management by anaerobic digestion gas recycling and mixing is used and recommended. One of the reasons of this is to provide adequate mixing of the digester content to prevent sludge accretion/stratification. It helps efficient utilization of the entire digester volume and uniformity of temperature.
Also, it disperses metabolic end products and any local over-accumulation of toxic material and to maintain intimate contact between substrate, bacteria and bacterial enzymes. In short, adequate mixing provides uniformity in digestion which is one of the ways to achieve a good digestion. Inefficient mixing on the other hand effects on process kinetics manifesting the decrease in effective reduction leading the digestion towards instability and failure causing reduction in gas productivity. In combination with temperature gradient due to inefficient mixing these reductions can be devastating.
Adequate mixing is most critical if digesters are to operate as designed. In this regard efficient gas recycling is of great help providing advantages of better control of scum collection at the top of the reactor sludge collection and removal of the bottom of the digester and additional mixing from natural gas evolution by convection currents.
Also recycling of anaerobic digestion product gases through the digestion mixed culture was expected to increase methane products because of additional methane fermentation from increased reduction of carbon dioxide. The sweeping action of the recycled gas was expected to accelerate removal of the gaseous products surrounding the microorganisms thereby minimizing any product repression.
From the experience of gas recycle it could be seen that as the gas recycle ratio is increased to an optimum level the opportunity of carbon dioxide reduction and sweeping of the gaseous digestion products out of the digester increases thereby stimulating methane production. However, as the gas recycling ratio is increased further the culture is increasingly saturated with gaseous end products thereby tending to repress or inhibit additional methane production.
The inhibitory effect of increasing gas recycle on methane production equal each other at the optimum gas recycle ratio which maximizes methane yield. Such participation of carbon dioxide in reductive pathways for additional methane has been demonstrated more recently. It has been shown that nearly 18% of excess methane production was originated by CO2 reduction reaction.
C. pH profile and pH effect:
In conventional anaerobic digestion of carbonaceous distillery effluent at the beginning of the acidogenic phase (phase I) the pH drops due to formation of organic acids. Later these acids are broken down and pH rises. This rise starts after 2 to 3 days of digestion and rises continuously till neutrality reaches to about 6.9 to 7.2 in about 10 to 15 days in batch digestion system. During this period (Phase-II) there is a rapid increase in methane production with corresponding decrease in volatile acids. In a classical study of digestion the initial pH was nearly 5.8.
It decreases linearly due to volatile acid formation to 5 in 3 days after which it again increased due to consumption of V A as substrate releasing NH3 by deamination of amino acids, which favours the growth of methanogenic bacteria. This increase occurred continuously till 11 to 12th day after that it was almost constant, total acidity increased up to first three days then it continuously decreased, total v.s. was also continuously decreased throughout the process.
The methanogenic bacteria have a limited pH range around maturity while the acidogenic bacteria exhibit intolerance of low pH values. Moreover, propionate, a volatile fatty acid and in-fact other transient may be toxic to the bacteria assimilating hydrogen in methane production as shown in the figure of rate limiting step. In a phase separated system, however, lower pH values may be possible in acidogenic phase where these may be advantageous to the associated microorganisms.
D. Effect of toxicity:
In anaerobic digestion of distillery effluent toxic substances can be present in the influent sludge as well as in the course of digestion. These toxic substances effect on anaerobic digestion to alter kinetics of organic (COD/BOD) matter degradation. The extent/magnitude of the toxicity depends on the nature and concentration of the toxic material.
In such conditions SRT will play significant role in process performance in order to minimize the adverse effects of toxicity on methanogenic microbial activity. Therefore, presence of propionic or transient toxic substances temporarily or permanently alters the kinetics of anaerobic digestion.
In case of presence of the inhibitory substance like formaldehyde to the extent of 100 ppm in distillery effluent has been demonstrated to cause an approximately 14% decrease in BOD removal efficiency at a SRT of 15 days and a 54% decrease in BOD removal efficiency at a SRT of 10 days. Likewise a transient toxic material like accumulation to high propionic acid in the digester requires to control SRT in methanogenic phase. Proper control of SRT also maximizes acclimation to toxicity.
E. Oxygen participation necessary or unnecessary?
Apparently oxygen plays important role by its presence at high tension. At high oxygen anaerobic digestion is impaired. Most probably because at high oxygen tension microbial metabolism of the flora begin to shift from anaerobic to aerobic and less methane is produced with a corresponding increase in cell biomass.
It appears that as optimal concentration of cell mass is required in anaerobic digestion trace amount of oxygen are required for biomass level maintenance. Air bubbling for this need is not desired as this oxygen demand is met by the nascent oxygen participation generated by cleavage of oxygen containing unsaturated materials in the digester. However, detailed analysis of this substitution of oxygen by oxygen containing compounds is scanty.
(iv) Survey Analysis of Three Distillery Effluent Treatment Process Technology:
A survey has been conducted by the appointed consultant of three distilleries which are using imported technologies.
These three distilleries are:
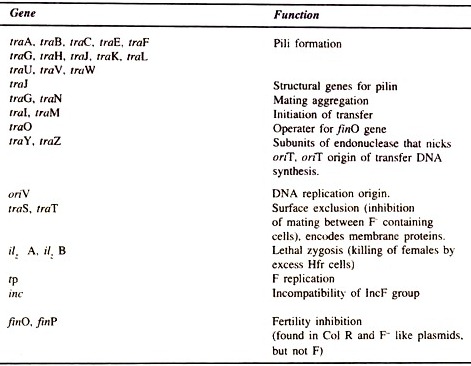
(1) Vam Organic Chemicals Pvt. Ltd. utilizing BIOTIM Process of Belgium,
(2) Rampur Distillery and Chemical Co. Ltd. using M/S AQUATECH Process, Thailand, and
(3) Ajudhya Distillery—using ADI-BVF Process of Canada
Their comparative data has been provided in Table 11.10.
The operational differences of these technologies arise because of the difference in knowhow. A comparative basic difference of these three technological knowhow in terms of cost, efficiency and few other technological parameters is presented in Table 11.10 as per data made available and approximated.
A. BIOTIM-NV- VOCE Process Technology:
This overseas technology supplied by BIOTIM-NV to VOCL is microbial system mediated anaerobic process. The process consists mainly of two microbial reaction stages/phases. The first digester has been called biological conditioning and control reactor (BCCR). In this bioreactor buffering and anaerobic hydrolytic reactions are carried out. The second digester has been called Methane Up flow Reactor (MUR) or Up flow Anaerobic Sludge Blanket Reactor (UASBR).
In this reactor anaerobic methane generation reaction is carried out. This process technology has been stated to have designed for installed capacity of 650 m3/day of distillery effluent to produce treated distillery effluent of BOD 100 mg/l and biogas 5.73 nm3 per annum on 300 working days and 3 shifts basis.
The essential features of this process as stated consists of receiving the distillery effluent at 85°C and cooling it to 40°C by plate heat exchanger. This raw material specification is given in Table 11.11. Next phosphoric acid is added to it as a nutrient. The addition of caustic soda is only required during the phase start-up.
This effluent with required amount of nutrient enters the BCCR and completely mixed by agitators. In this bioreactor the acidogenic bacteria break the complex organic carbonaceous molecules into simpler molecules of volatile organic acids suitable and susceptible to biomethanation reaction.
This conditioned effluent from BCCR is desludged in parallel plate separator (PPS) and part of the sludge is recycled to BCCR. The liquid effluent from PPS is mixed with requisite chemical to adjust pH adequate for methane formation reaction and pumped to MUR through the distribution system at the bottom of the reactor.
In MUR the methanogenic bacteria act on smaller molecules of volatile organic acids to produce methane containing biogas. Clear treated effluent overflows to the effluent holding tank. Part of this treated effluent is recycled and the rest is sent to the secondary treatment system for further treatment. Biogas is sent to captive boiler with the help of a blower for use as fuel replacing furnace oil.
Excess gas if any may be faired. Utilities consumption for treatment of 680 m3/day of distillery effluent has been stated to be as follows: caustic soda only during start up, H3PO4– 60 kg/day, cooling water 60 m /h (circulating) and Power-1050 KWH/day.
Why BIOTIM N V Technology has been selected by VOCL? After thorough investigation of similar treatment processes for distillery effluent VOCL observed and stated that the two stage reaction system of BIOTIM is very efficient in BOD reduction and biogas production. It is also claimed to be capable of adjusting to variations in operating conditions.
Also it is claimed that this technology has the following salient features:
i. Two stage digestion system for rigid control of microbiological reaction with minimum packing.
ii. Lower capital investment because of lower packed volume.
iii. Special design feature of packing dominates clogging of foaming.
iv. Reduction of BOD and COD to the extent of 80% and 70% respectively.
B. AQUA TECHNO-RDCL process technology:
RDCL’s Envisaged Scheme: RDCL has envisaged for treatment of 1000 m3/day spent wash effluent from the cane-molasses based distillery having BOD level of 40, 0000 to 50,000 ppm to generate 3000 m3/day biogas rich in methane having calorific value of 5000/5500 k. cal/m3 which can be used for generation of heat/power by adapting the anaerobic digestion technology of M/s AQUA TECHNO, Bangkok. RDCL also envisages utilizing methane generated from degradation of spent wash for replacing its furnace oil requirements in its SSP granulation plant, as a fuel in its boiler and partly for generation of power.
Process Biotechnology:
The process involves anaerobic digestion of the dilute distillery effluents with constant agitation in presence of limited supply of air. The methane gas generated during the digestion process is scrubbed and stored in gas holders. The spent wash has been envisaged to pass through trickling filter for deodourising and discharged in the channel for dilution. The treated sludge from the tank has been planned to be sun dried and used as filler/fertiliser.
C. Analysis of the comparative data of the three technologies:
The digestion of distillery spent wash/molasses slop was studied nearly fifty years ago. This and other studies used conventional stirred tank digesters resulting in SRT exceeding a week. Typical degradation was seldom higher than 3.5 m3. M-3d -1. Even by the use of anaerobic contact processes the anaerobic digestion of distillery effluent could not be improved to any great extent. HRT and hence reactor volumes were nearly the same order of magnitude.
The application of the UASB/MUR to diluted molasses based distillery effluent as used by Vam Organic Chemical Ltd. (VOCL) and to other process technology is restricted to low or medium strength effluents while initial concentration of distillery effluent COD may be as high as 100 to 120 gsr1. Therefore suitability of such effluent digestion process technology in sludge bed digester still remains under scope of constraint.
(v) A Typical Process Design Information:
B. Process outline of methane up flow reactor (MUR):
Distillery effluent is first conditioned by increasing its pH with addition of lime and chemicals (nutrients) and then if necessary cooled to 40-45°C. The conditioned effluent is fed to first stages reactor BCCR where degradation of organic matter in the effluent into volatile fatty acids (VFA) take place by liquefying and acidogenic microbial flora (bacteria).
Effluent of BCCR is passed through pps (parallel plate separator) and separated sludge is recycled back to BCCR. Effluent from pps is fed to the two MUR where VFA are converted into methane gas by methanogenic bacteria. Effluent of MUR is passed through cross flow pps and goes to secondary treatment.
Coal Processing Effluent Water:
1. Forewords:
The present energy generating system in rural and most industrial areas in India is coal. Coal has been expected still to be an alternative energy source in place of oil. About 7308 million tons of coal reserves was confirmed in 1986 and is omnipresent in many countries. The smoke of coal combustion like biomass burning in rural areas creates an unhealthy environment to local dwellers. Also, coal has the problem of handling and S02 produced by its combustion leads to acid rain.
Many coal based industries like steel, fertilizer, carbon black etc. are in operation in Eastern India particularly in Durgapur industrial belt of West Bengal. Also the petroleum refinery at Haldia has constituted another dimension to the industrial development of Eastern India.
All of these industries generate large volumes of wastes/residues and waste waters. These wastes and waste wasters are vulnerable to cause pollution hazards in the locality or local portable/ usable water. They need adequate processing for pollution abatement prior to their recycling or disposal. If they are disposed of without giving adequate treatment/care they are likely to create environmental pollution either by purification or by biodegradation.
Liquid waste effluents discharged from these industries ranges between 0.03 to 0.4 M3/ton of coal processed. Characters of these waste waters/liquid effluents are toxic in terms of pH, colour, dissolved solids, phenol, free ammonia, thiocyanate, cyanides, sulfide, BOD, COD, etc.
2. Hazardous components and toxicity:
The major toxic components in the liquid effluent of these industries are phenol, cyanides, thiocyanates etc. Toxicity features of few of them are presented in Table 11.13. When humans unknowingly consume water polluted with phenol they have been reported to become ill due to diarrhoea, mouth sore, dark urine and burning mouth.
The toxic effects of phenol may not be so acute with regard to human being but its presence in traces in potable water is objectionable, simply because upon chlorination it forms chlorophenols, which impart unpleasant taste. Also the basic phenolic compounds are reported to be carcinogenic in nature. Phenol toxicity to fish life has been recognised as early as 1928. When 8″ carps were exposed to phenol concentration of 400 mg/l within 1hr they have been killed.
Cyanide is an extremely toxic substance. In mammalian systems hydrocyanic acid prevents the process of oxidation in the tissue cells. It paralyses the respiratory centre of the brain cells. The lethal effect of cyanide ions is due to the inability of the tissue cells to utilize oxygen. Cyanide is known to be an inhibitor at the terminal oxidation of the cytochrome (cytochrome oxydase).
Cyanide forms a stable complex with cytochrome oxidase. This complexion results in loss of the enzyme activity resulting in death. Hydrocyanic acid is toxic at 0.3 mg/l of the air inhaled. The lethal dose of the toxicant is 1.0 mg/kg body weight. The fatal dose for animals is 9.0 mg/kg body weight.
Fish has high sensitivity to cyanide. Minnows, catfish and carps behaved sick at a concentration of 0.3 mg/l. Pottassium cyanide at concentrations between 0.03 to 0.12 mg/l has killing effect on gold fish when exposed to the toxicant for 3-4 days. In flowing water, containing cyanide above 0.03 mg/l cent per cent chance of survival of white carps is uncertain.
Thiocyanate at concentrations above 15 mg/100 ml of blood is critically dangerous. Chronic adsorption of thiocyanate may cause dizziness, skin eruptions, running nose, vomiting, nausea and mild to severe disturbances of the nervous system. Thiocyanate containing water upon chlorination or irradiation releases the cyanide by splitting the sulphur moiety. It inhibits the adsorption of iodine by thyroid gland. Ammonium thiocyanate is reported to have lethal effect on average spotted shellfish at 280-300 mg/l while goldfish exposed to 1600 mg/l concentration has been reported killed in 24 hours.
Free ammonia has been found to be toxic to fish life even at concentration level up to 2.8 mg/l. The toxicity of ammonia is due to the unionized ammonium ion concentration which increased in solution with increase in pH.
Waste water from the coal carbonization industries, when it is fresh, has a straw yellow colour. The colour is intensified upon storage and exposure to atmospheric conditions and aeration. Several explanations have been given for the development of this colour.
The catechol present in the waste under alkaline conditions oxidized catechol. The wastes contain iron which can also form colouring complex with thiocyanates, cyanides etc. to impart colour to the waste. The chemistry of the colour development is yet unknown. However, the colour development during exposure to air and light has been shown to exhibit toxicity of algal population above 600 units.
Very little information is available with reference to the sulphide content in the coal carbonization effluents except that in the case of LTC process it is between 67-74 mg/l. Soluble sulphide in water reacts with hydrogen ion concentration to form HS or H2S. The toxicity of sulphide is derived from the H2S rather than the sulphide ion. The lethal concentration of sulphide for fish life is reported to be between 0.032 and 0.355 mg/l.
The BOD to COD ratio of the wastes from different coal carbonization processes works out to be between 0.5 to 0.9 indicating that it is amenable for biological treatment. Several attempts have been made to treat the waste after dilution with domestic sewage.
3. Major liquid effluents origin in coal processing routes:
Both conventional and non-conventional coal processing routes have been provided by scientists and technologists (Fig. 11.7 and Fig. 11.8). Conventionally coal has been processed as a raw material for energy source and to produce coke for many industries. In recent years its non- conventional bio-processing routes have also been known (Fig. 11.8).
In both routes the need of coal processing has been to serve the following purposes:
(a) Produce low sulfur coal
(b) Reduce smoke generation
(c) Reduce air pollution
(d) Reduce health hazard
(e) Reduce COD/BOD
(f) Higher heat generation from coal and
(g) Obtaining possible useful metabolites/chemicals.
(i) Conventional:
Conventional route of coal processing is shown in Fig. 11.7. However, conventional processing of coal is expensive. For partial reduction of the expense possibility of unconventional microbial biodegradability of pollution generating liquid effluents has been shown. Bio-despiritization of coal by microbial processing is one such approaches. Bio liquefaction and bio desulfurization of coal are other approach.
It is necessary to identify potential microbial strains for such purposes. However, microbial processing of coal for bioremediation of hazards is associated with both merits and demerits (Table 11.14). Comparative process conditions of both routes are given in Table 11.15.
(ii) Non-conventional: Bioremediation:
1. Aspects of bioremediation:
(i) Potential Microbial Strains:
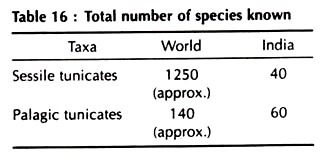
Non-conventional bioremediation routes make use of potential activity of microbial strains in degradation of pollution generating components in coal and coal effluents. Some eubacteria and archaebacteria have been found to be highly potential in bioprocessing of coal for bioremediation. These microorganisms accelerate the oxidation of pyrite producing ferric sulfate and sulfuric acid. Few potential microbial strains of coal bioprocessing are given in Table 11.16.
(ii) Biodepyritization Strategies:
As in Table 11.17 biodepyritization strategy may vary depending on conditions. In heap or percolation bioleaching the actual composition of microbial population is still not clearly known. The disadvantage of this method is long time required for desulfurization. However, its advantage envisaged is that thermophilic operations (50-60°C) possibly accelerate rates at elevated temperatures.
Treatment without dilution with domestic waste has also been reported but by supplementation with phosphorus. The influent phenolic concentration has been found to influence the aeration period. Pilot plant studies have showed that the waste water from steel mills (coke ovens) could be treated in aerobic activated sludge plants with an organic loading of 0.716 lb/lb of sludge/per day.
Ammonia concentration above 4000 mg/l is reported to inhibit severely the biological activity of the sludge. Apart from ammonia, thiocyanate, cyanide, sulphide oxidized polyhydric phenols, calcium and chlorides also affect the biological process. Biological treatment of coal carbonization waste is widely practiced in Britain independently because in most cases, discharge municipal sewage is not available for making enough dilution.
Waste water containing phenols, cyanides, thiocyanates and ammonia is amenable for biological degradation; there are many inherent factors in bioremediation by biodegradation of organic pollutants of coal. These factors and their consequences are listed in Table 11.18 to 11.20.
(iii) Fundamentals of Bioremediation:
Phenolytic microorganisms may belong to bacteria, archaebacteria, yeast and fungi. The metabolism of the aromatic ring can take place under anoxic as well as aerobic condition. Considerable amount of information is available on the oxic metabolism of aromatic compounds. Biologically phenol forms catechol. The enzymes involved in biological pathways are hydroxylases, oxygenases, dehydrogenases and aldolases. These enzymes are subjected to feed back inhibition. The substrate itself acts as substrate inhibitor to the whole cells.
In bioremediation biological oxidation may occur by the different routes:
(i) Removal of electrons by transformation of metallic form of ferrous iron to ferric
(ii) Removal of hydrogen as in the case of alcohol to acetaldehyde and,
(iii) Addition of oxygen as in the case of oxidation of carbon to carbon-dioxide.
The participation of oxygen in the reactions is that of the electron from the terminal oxidation stage. Oxygen is required in certain other enzymatic reactions. The steps in the biochemical transformation of the aromatic involve the introduction of molecular oxygen.
One atom of oxygen is inserted into the substrate and the other atom of the molecule is reduced to water as given below:
RH + O2 + 2 (H) ——> ROH + H2O
Here RH represents the aromatic molecule. The aromatic ring is attacked by the enzyme “oxygenase”. For ring fission both atoms of one molecule of oxygen are incorporated into the hydroxylated ring. In the oxidation of a simple aromatic molecule, in addition to the electron transport chain reaction, molecular oxygen is also required for the opening of the aromatic ring. A variety of microorganisms have been found to metabolize cyanide and phenolic compounds. Most of them have metabolic pathways leading to the formation of amino-acids.
(iv) Bioprocess Engineering Analysis:
The scheme of detoxification of phenolic waste water at pilot plant level bioremediation is shown in Fig. 11.9 in which Candida tropicalis was used. Inoculum need in the process was developed as described in Fig. 11.10. Considering the schematic diagram of Fig. 11.9 and operation of completely mixed ASP system, the material balance of the substrate detoxification is given by:
(v) Bioremediation Reactor Studies:
In recent years considerable increase in the environmental impact of waste waters/liquors from coal processing systems is evident. This is arising from the coal gasification and liquefaction processes which are still under development. Among the compounds that are the greatest bio-hazardous in coal derived waste liquors as discussed in preceding sections are phenols thiocyanates, ammonia and cyanides. Bioremediation by simply eliminating low level of phenol from waste waters through phenol fermentation has been used most frequently.
The general strategy to bioremediation of waste liquors is the application of highly evolved symbiotic populations of microbial flora (aerobic or anaerobic) in a classic reactor design so that all hazardous polluting compounds are degraded in the process. For a long time the basic reactor design continued to be the simple activated sludge aeration vessel as used in ASP followed by a clarifier. Many investigators have made systematic studies to ascertain the optimum operation parameters for the activated sludge in a continuous stirred tank bioreactor (CSTBR).
Techniques of determining limiting reactants, minimum retention time for biodegradation of limiting reactants and understanding of the relative inhibiting effects of various compounds upon other compound degradation rates for different feed rates and concentrations in three different conventional reactor configurational modes CSTBR, a three phase (FBBR) was interesting.
It could elucidate relative merits of each bioreactor for accomplishing bioremediation in terms of degradation of a given daily discharge of phenol bearing waste water having common synthetic feed composition. In all bioreactors the general equation used in comparison of degradation rates (DR) was as given below.
In which QL is the volumetric flow rate. VR is the volume of the reactor and Ci and C are concentrations of phenol in inlet and at any time. Typical experimental results using active symbiotic bacterial population could indicate that if the volumes of the bioreactors were about equal, the CSTBR could treat 2 VR (volume/ day), the PBBR 5.0 VR and the FBBR 8VR (undiluted volumes). These information’s provided preliminary indication on the suitability of FBBR in the effective bioremediation of phenolic hazards from coal processing liquid effluents.
More recent study on phenol fermentation in a small continuous STR bioreactor using pure culture of Pseudomonas putida strain (ATCC 17484) could develop rate equations of phenol biodegradation, the rate of intermediate inhibitor accumulation or degradation and the rate of cell biomass production. The phenol biodegradation rate constant for pure culture system was about 0.4-1.2 while in mixed sludge culture system kphe was about 1.9 x 10-2 ± 8.8 x 10-3. In sludge it is determined using acridine orange direct counting (AODC) procedure.
(vi) Useful Chemicals from Bioremediation Pathways:
A. Meta-ortho pathway:
Coal bioprocessing and bioremediation of coal processing effluents produce several important metabolites by transforming hazardous components of coal. One example is the formation of useful amino acids through meta-ortho bioremediation pathway (Fig. 11.11). In this pathway 2, hydroxymuconic semialdehyde has been stated to be an important intermediate. However, in terms of its usefulness it has not been characterised as yet.
B. Organic sulfur removal pathway:
Bioremediation for microbial desulfurization of coal is to remove sulfur cleaving C-S bonds in model compound. It resembles coal organic sulfur. Dibenzothiophene (DBT) has been used most extensively as model compound. This process causes liberation of sulfate without oxidation or degradation or organic carbon matrix.
A possible pathway of this sulfur removing bioremediation involves steps as available elsewhere. At international level lot of research is in progress for cloning of responsible genes for this pathway for more efficient desulfurization and ability to transfer such genes to other organisms in order to develop potential technologically redesigned cells.
Food Processing Effluent Waters:
1. Forewords:
Food industries like canning, sugar mill, rice mill, distilleries, live stock and meat processing dairy industries, fruit and vegetable processing etc. generate a big quantum of by-products as waste and waste water. Since food processing plant waste waters may contain a variety of materials their compositions and contamination loads vary greatly. However, on the whole food plant waste waters can be broadly classified according to the nature of their impurities and pollution strength, which determines what treatment method, may be appropriate/suitable.
In food plant waste water materials present may vary from coarse floating or sinking solids to colloidal particles. Also, beyond this size limit there are substances in true solution. Water insoluble liquids like oils, fats and solvents may be present also.
The impurities present in all these materials are of either chemical or of biological nature. Besides hazardous metallic contaminants water and waste waters usually contain organic nitrogenous material. In waste water purification microbial nitrogen rich carbon poor (or low C/N ratio) environments of denitrification process may serve as key to clean water. When C/N ratio is high causing short circuiting nitrogen cycle the objective of waste water treatment is defeated.
2. Treatment of waste and waste water from food industries:
Nearly all thermal processes in food industries use steam for various operations. For example, in the canning of food exhausting, blanching and sterilization/pasteurization are the major operations involving utilization of steam. The range of steam requirement in these operations is given in Table 11.21.
To meet this requirement steam is produced in a boiler to maintain a line pressure of 100-125 psi. Steam production, flow and consumption is expressed in terms of boiler horse power (1 boiler horse power = 7200 k cals/hr). For a process lasting an hour a total of approximately 300 lbs of steam of 8.7 boil horse power per hour is consumed. It corresponds to energy requirement of about. 6.5 x 104 k cals/hr.
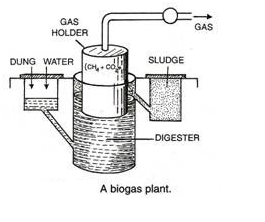
A part of this energy can be recovered from the generated refuse waste of the food industry through its anaerobic bioconversion in biogas plant into methane. Essentially a single stage biogas plant consists of two units. First, a digester with an inlet into which the waste materials are introduced in the form of slurry.
In the digester bioconversion of refuse waste is carried out at 35-40°C. The second unit is a gas holder to collect biogas containing 60-65% methane as combustible component the rest being mostly C02. In a typical anaerobic digestion process to generate biogas in the digester the complex organic waste food materials are degraded in a sequence of two steps mediated by two different types of mixed microbial flora.
In the first liquefaction and acidification step acidogens like acetic acid bacteria play role for nearly seven to ten days. Liquefied acidic materials are acted upon by methane forming organisms in the second step and digestion is completed by another five to eight days. Improvements of this basic process biotechnology of biogas generation from food wastes have been made and thereby digestion time has been reduced simultaneously methane productivity has also been increased to a great extent.
A list of computed daily availability of biogas from food waste and its energy equivalent is given in Table 11.22. Although these waste resources are exploitable but it appears that these important resources have not been exploited for small scale bioconversion into methane.
The waste water from food processing industries like dairy use the principle of permeability of membranes in whey separation and treatments. Also for purification and fractionation as well as in waste disposal and by product recovery from waste liquid effluents membrane processing has been very useful.
Membrane treatments primarily provided major advantage in retaining natural aroma and fresh flavour. That is why in treatment of fruit juices and other beverages membranes have been favoured extensively. In beer and alcohol distilleries clarification and removal of yeast by microfiltration (MF) is now in practice.
Tannery Effluent Waters:
1. Forewords:
Tannery effluents are generated in various steps in producing leather from raw skins and hides. After deskining and setting tanning objectives various process steps which are involved in the manufacture of finished leather include, curing, soaking, liming, dehairing/unhairing, bating, oiling, pickling, dyeing and finishing. Many chemicals are used in these processing steps. Among the chemicals used chlorides, sodium carbonates, ammonia, calcium and sodium dichromates, sulphuric acid etc. are the major ones. In finishing step organic dyes are employed.
It has been estimated that per kilogram of finished leather nearly 35 liter of effluent water is generated. This waste water needs adequate treatment before disposal because untreated tannery waste waters corrode sewer lines, cause ground and surface water pollution and imparts odor problems.
For abatement of these adverse effects tannery effluents must be treated to safeguard the receiving bodies/sink and surface and ground water sources. As shown in Fig. 11.13 animal skin has several layers. Among these layers only inner corium layer is useful in leather making. The corium is made up of collagen (C102 H149 N31 O38). The objectives and processes in tanning indicating waste water resources are described below.
2. Objectives of tanning:
i. To strip off two outer layer from the skin to subject the corium to the action of tanning agents.
ii. When subjected to the action of tanning agents it undergoes a transformation and becomes insoluble in water, tough and flexible as highly durable leather.
3. Processes in tanning and waste water resources:
(i) Curing:
All Skins
Covered with numerous bacteria
When an animal is alive, these bacteria have little effect on the skin.
Slaughtered or dies
Proteolytic bacteria quickly attack the skin and reduce its value and utility by damage.
Damage prevention Skin is cured with salt or by drying in air and a combination of both.
It removes
Free water from skin, reduces bacterial growth——– > Waste water.
F. Fat liquoring and dyeing:
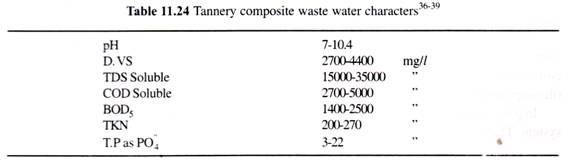
Tanned leather is treated with emulsion of sulphonated castor oil or dyed with different colours to produce finished leather and generating waste water (characteristics in Table 11.23). However, the waste waters from various steps having nature as given in Table 11.23 when reaches the treatment plant the tannery composite waste water characteristics become as given in Table 11.23 and biological composition of settled and filtered composite tannery waste shows value, as in tables, 11.24 to 11.26.
4. Treatment methods of effluent waters:
Wastewater from tanning industry can be treated in the following steps: Segregation, Primary treatment, and Secondary treatment.
(i) Primary Treatments:
The immense difficulty felt with tannery effluent is its ever changing composition and flow rate. Therefore, to have a proper control on effluent treatment, the different types of operational discharge are stored in a storage tank for a period of about 12 hours to equalize and to facilitate neutralization of acid and alkali waste.
The removal of suspended solids further can be facilitated by the use of coagulants like alum, ferric chloride, sulphuric acid, etc. Flocculation with ferrous sulphate and polyelectrolyte combination of tannery effluents is significant in lowering of (i) total solids (ii) BOD (iii) sulphide content (<5 ppm).
In general some initial pretreatment processes are carried out to facilitate the overall treatment system. These can be highlighted as follows:
A. Segregation:
Effluents from different tannery operation are highly varying in nature in pollution load. Therefore, the wastewater from such an operation which contributes to a great extent to the pollution load should be treated separately to reduce the intensity of pollution. It is better to segregate soaking effluent containing a very high quantity of chloride. The segregated effluent from soaking operation can be evaporated in a solar evaporation pan.
B. Removal of sulphide:
The suspended matter like lime, hair, fleshing, etc., can be removed along with toxic trivalent chromium by simple mixing and sedimentation which can reduce about 70-80% suspended solids and about 30-40% BOD of the tanning waste. But before doing so, it is advisable to go for removing sulphide from the lime liquor first, otherwise the sulphide removal after mixing acidic and alkaline effluent will need to ventilate with considerably greater volume of effluent.
C. Recovery of chromium:
Large amount of chromium chemicals are used in the process. Fortunately, however, most of the chromium used is of trivalent nature. Hence its removal or recovery system is relatively simple. There are two types of simple possibilities of recovery of chromium for its reuse. The first one is the simple filtration of chrome tannage effluent to remove the hide fibres and use the liquor for the initial mild pretannage in the subsequent batches. This helps in reducing the discharge load of chromium in the effluent.
The second process involves precipitation of chromium after collecting the effluent from chrome tannage separately by means of alkalis. The chromium hydroxide precipitate can be re-dissolved by sulfuric acid and mixed with sodium sulfate to produce tan liquor for use in the next tanning batch. The recovery of hydroxide may be made with filter press or centrifuge.
D. Removal of suspended solids:
Tannery effluent and the large suspended solids content are very synonymous. As already said, the suspended solid consists of lime, lumps of ‘stringy’ flesh and hair. These small lumps can be easily removed by screening.
(i) Secondary Treatment Processes:
Normally an aerobic process is used to reduce the organic content of the effluent i.e., BOD and COD. However, because of the high values of these components the energy consumption in the process which is necessary for the supply of oxygen required for the oxidation of the organic components is quite high. Presently a combination of anaerobic and aerobic method is favoured. This involves passing the effluent through an anaerobic filter followed by the aerobic process. Aerobic process may be chosen by anyone among the many systems being used as outlined briefly in the following sections.
A. Attached growth process:
Trickling filter is one type of attached growth process. Here the microorganisms grow firmly attached to solid support while the waste water flows through the packed bed of solid support. In this way, microbial growth continues due to the sprinkling of waste water on to the filter bed thereby forming bacterial slime augmenting day by day creating ultimately such a situation when the inner layer is deprived of oxygen weakening the attachment with the filter bed allowing a part of the slime to slough off. Most of the BOD is removed and the residual solids are settled out in the form of stabilized sludge.
ADVERTISEMENTS:
(a) Merits and Properties of Plastic Media:
The basic process has been modified to enrich the performance of such a filter. The greatest change from the basic pattern is the use of plastic media in place of crust rocks. Trickling filter with plastic media weighs much less thereby the bed depth can be increased utilizing a minimum land area.
Plastic packing is of greater specific surface enabling a high strength liquor treatment with higher efficiency due to high voidage and here lies the marked difference with mineral media. Another reason for the replacement of mineral rocks is the high building costs of deep packed mineral beds.
Their merit is also in removing more BOD and greater open structure facilitating air circulation throughout the bed depth. They do also satisfy the already existing quantities of mineral rocks surpassing the merits of the latter. Moreover, the bacterial slimes produced in plastic media is biologically similar to that on the other one. No objectionable report on the settle-ability of the sludge from such a media is yet available. With the decrease of specific surface, the phase separation decreases. It is known as low-age sludge or, in the other sense, these are not stabilized.
(b) Rotary Biological Contactors:
It is a novel modification to the traditional bio-oxidation techniques to achieve a high operational performance by retaining a high concentration of the active biological solid in the oxidation zone for a long period of time. In this process a series of flat circular plastic plates are arranged to rotate on an axis in such a way as, to submerge it partially.
Bacterial food is produced in the immersed section whereas the outer zone acts as a source of oxygen. This system is more reliable in operation than the other one. Nowadays an extended form of rotary biological contactors is employed in a perforated rotating drum packed with random plastic packings.
B. Suspended growth process
(a) Activated Sludge Process:
This process is a fundamental suspended growth process, accounts for most of the biological purification in sewage treatment plant because of its less working area than the trickling filter process. The basic principle of this operation is the microbial growth in an aerated system containing bio-degradable organic food. The microorganisms grow and form floes mixed with some organic and inorganic. When aeration is stopped after the consumption of food the mixed floes will aggregate and settle down leaving a clear supernatant of low BOD and suspended matter.
(b) Usefulness of Combined Treatment of Tannery Effluent and Settled Sewage:
Tannery effluent after primary treatment is better to mix with sewage. It acts as a dilution to the concentration of tannery effluent with respect to the presence of toxic substances like Cr III as well as some bactericide used as preservative in leather processing.
These substances used in tannery and present in wastewater might be inhibitory to treatment in an industrial biological treatment plant but dilution with sewage decreases their hindrance. The activated sludge process otherwise is too easily upset by the toxic substances present in the undiluted tannery effluent.
That is why the activated sludge plant is very unlikely to be suitable for such effluent treatment. The large volume of biological solids present either as bacterial slime in trickling filter or as activated sludge in such a treatment process helps also to absorb such toxic substances along-with certain other organic and inorganic to the extent of 0.5% of the dry weight of fresh sludge thereby reducing the accentuating mark of such materials potent of adverse effects present in industrial pollutants.
Obviously, the pollutants this way change their form of pollution, namely from liquid to solid creating the problem of the sludge disposal. Tannery wastewater contains biodegradable organic matter but it is a necessary fact that the tannery discharges do not contain all the nutrients needed for biological growth in the required ratio that is BOD: N: P = 100: 5: 1.