ADVERTISEMENTS:
The below mentioned article will highlight the three important techniques of biotechnology.
The three important techniques of biotechnology are: (1) Recombinant DNA Technology (Genetic Engineering) (2) Plant Tissue Culture and (3) Transgenic (Genetically Modified Organisms).
Technique # 1. Recombinant DNA Technology (Genetic Engineering):
Genetic engineering deals with synthesis of artificial gene, repair of gene, combining of DNA from two organism (recombinant DNA) and manipulating the artificial gene together with the recombinant DNA for the improvement of microbes, plants, animals & human being.
ADVERTISEMENTS:
Genetic engineering or Recombinant-DNA Technology involves cutting and pasting of desired DNA fragments It is based on two important discoveries in bacteria:
(i) Presence of plasmids in bacteria which can undergo replication along with and independent of chromosomal DNA
(ii) Restriction endonucleases which can break DNA at specific sites. They are appropriately called molecular scissors.
The science of recombinant technology took birth when Cohen and Boyer (1973) were able to introduce a piece of gene containing foreign DNA into plasmid of Escherichia coli. Berg (1979) was able to introduce a gene of SV-40 into a bacterium with the help of lambda phage. Berg is often considered “father of genetic engineering”.
Basic Tools of Genetic Engineering:
ADVERTISEMENTS:
Three types of biological tools’ are used in synthesis of recombinant DNA. These are as follows:
1. Enzymes:
These include lysing enzymes (e.g., lysozyme), cleaving enzymes (e.g., exo-nucleases, endonucleases, restriction endonucleases), synthesizing enzymes (e.g., reverse transcriptase’s) joining enzymes (e.g., ligases) and alkaline phosphatases.
2. Vector or Vehicle DNA:
The DNA used as a carrier for transferring a fragment of foreign DNA into a suitable host is called vehicle DNA or cloning vector or gene carrier (e.g., plasmids, bacteriophage DNA, cosmid – an artificial plasmid, phagemid/phasmid, artificial chromosome vectors).
3. Passenger DNA:
It is the DNA which is transferred from one organism into another by combining it with the vehicle DNA (e.g., complementary DNA- cDNA, Synthetic DNA— sDNA, random DNA).
(a) Complementary DNA:
The desired gene is prepared from mRNA by reverse transcription process and the product is complementary DNA (cDNA).
ADVERTISEMENTS:
(b) Synthetic DNA:
The desired gene is synthesized using a computerized machine called DNA synthesizer or DNA gene Machine. This machine can synthesize gene of more than 1000 nudeotides length according to a predetermined sequence. If the number of desired genes is not enough for cloning, the gene is amplified using polymerase chain reaction.
(c) Random DNA:
The desired gene is isolated from the total genomic DNA of an organism. To isolate a gene, the genomic DNA is cut into number of pieces with the help of restriction endonuclease. The pieces of DNA are separated on the basis of their length using electrophoresis and later on can be used for cloning. Artificial or synthetic chromosomes from bacteria and yeast called BACs and YACs, respectively are more efficient for eukaryotic gene transfers (Fig. 1.2).
Recombinant DNA Technology:
It involves the following stages (Fig. 1.3):
1. Isolation of DNA segment:
The DNA to be used as vehicle or passenger is taken out from the cell by lysing it with a suitable enzyme. The DNA is isolated from other cell contents. Then both vehicle and passenger are cleaved by using the same restriction endonuclease so that they have complementary stickly ends.
2. Formation of Recombinant DNA:
ADVERTISEMENTS:
The complementary sticky ends pair and their ends are sealed with ligase. This produces a recombinant DNA (or chimera).
3. Production of Multiple copies of Recombinant DNA:
Next step in the process is production of multiple copies of recombinant DNA.
4. Introduction of Recombinant DNA into Host:
ADVERTISEMENTS:
E. coli, Bacillus subtilize and yeast are used as hosts for the recombinant DNA. The bacteria to be used as hosts should be without plasmids. Three methods are used for introduction of recombinant DNA into the host.
(a) Transformation:
It is the process by which a cell takes up naked DNA segment from the environment, incorporates it into its own chromosomal DNA.
(b) Transduction:
It is the transfer of DNA from one organism to another through a bacteriophage.
(c) Vector less Gene Transfer:
ADVERTISEMENTS:
Gene transfer can be affected by certain means that do not use vectors. It may be done by microinjection needles and gene gun.
5. Screening of the Transformed Cells, (i.e., Selection of clones with recombinant DNA):
The next step is to determine which cells harbour the recombinant DNA molecule containing the gene of interest. The desired clones can be selected on the basis of the presence of the vector or of the inserted gene itself. For example, some plasmid vectors confer resistance to an antibiotic.
Another approach is to determine which cells bind RNA that is complementary to the gene of interest or synthesize protein encoded by it. Clones containing recombinant DNA are stable for at least several hundred generations.
Reporter Gene for Gene Monitoring:
One of the technical problems encountered in attempts at gene transfer is knowing whether a particular gene has actually been introduced into a new host cell and. if transferred, whether it is directing the synthesis of protein.
To overcome this problem, reporter gene have been developed, which can be transferred to the plant cell with the help of Agrobacterium. For gene monitoring, the reporter gene must be linked to a promoter. When the promoter is switched on, the reporter gene is activated.
ADVERTISEMENTS:
Three reporter genes currently in use are GUS, the Escherichia coli β-glucuronidase gene; the luciferase gene, which is obtained either from fireflies (Photinus pyralis) or the marine bacterium Vibrio harveyi; and the gene for a green fluorescent protein from jellyfish.
The various steps involved in a gene transfer are given in the figure 1.3:
Agrobacterium Tumefaciens—A Natural Genetic Engineer of Plants:
The most popular organism for the transfer of foreign genes into plants is Agrobacterium tumefaciens, a soil-dwelling bacterium that infects a wide range of dicots, typically gaining through wounds. Agrobacterium tumefaciens induces the formation of tumors, called crown-gal tumors on the plant by transferring a specific region, the T-region, or T-DNA, of a tumor-inducing (Ti) plasmid to the host’s nuclear DNA.
Agrobacterium tumefaciens, with its Ti plasmid, is a powerful tool for genetic engineering of dicots. The tumor- promoting genes on the T-DNA can be removed and replaced by foreign genes. Infection of a plant with Agrobacterium containing these engineered plasmids will result in transfer of the foreign genes into the plant’s genome.
Transgenic plants (plants containing foreign genes) obtained through the use of plasmids will transmit the foreign genes to their progeny in a Mendelian fashion. Considerable progress has been made in applying this method to the transfer of agriculturally useful genes. These include genes for resistance to insects, herbicides and viruses. In some cases, genes that mediate pathogen resistance in one species of plant are being identified and transferred into dise-susceptible species.
In an extreme case, a gene from the bacterium Bacillus thuringiensis (the BT gene) has been transferred into plants. The BT gene codes for a protein toxin that specifically kills lepidopteran larvae-that is, the larvae of butterflies and moths. Plants expressing this gene are resistant to damage from caterpillars.
Genetic Engineering in Animals:
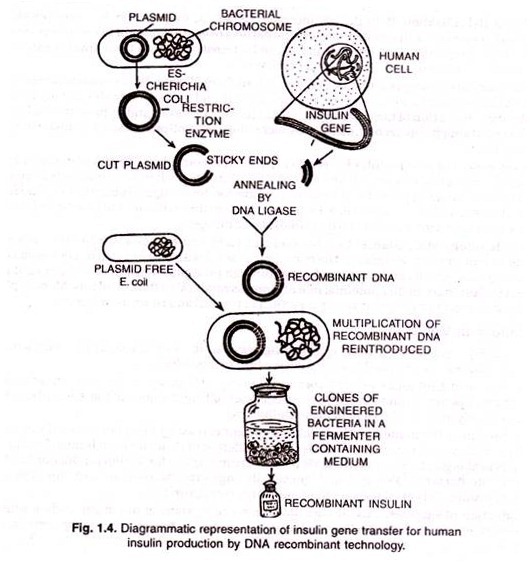
Procedures, parallel to practiced in plants and described above, are followed in animals also for recombinant DNA technology. An illustrated account of the mechanism of transferring insulin gene from human cell to E. coli for human insulin production is shown in figure 1.4.
Using genetic engineering techniques similar to those described, genes from a variety of plants and animals, including humans, have been introduced into bacteria. The fact that eukaryotic genes can function inside prokaryotic cells is one of the strongest arguments for the unity of life at the molecular level. The seemingly only limitation of the cloning technique is the cleverness of the microbial geneticist in choosing appropriate media for selecting the hybrid plasmid of interest.
Hazards of Genetic Engineering:
Several hazards (dangers) are associated with recombinant DNA technology.
The main dangers include:
(1) Spread of New Diseases:
New dangerous forms of microorganisms can be developed through recombinant DNA technique either accidentally or deliberately. Escape of such microorganisms from the research laboratory through drainage, laboratory glassware, laboratory personnel etc., may lead to the spread and origin of new diseases, which may pose a serious problem. HIV (AIDS Virus) is believed to be one such product.
(2) Effect on Evolution:
Nature has provided several barriers for exchange of DNA between prokaryotes and eukaryotes. Recombinant DNA technology permits exchange of DNA between these two classes of organisms and thus interferes with the natural process of evolution.
(3) Biological Warfare:
There is always a possibility that some unscrupulous countries may use genetic engineering technology for biological warfare. In such warfare, disease-carrying microorganisms can be used against the enemy. This is bound to lead to disaster. Thus genetic engineering has several demerits also. Many of the results achieved through genetic engineering can be achieved through other less hazardous techniques.
Cloning:
Two exactly similar or carbon copies of organisms having same source of origin are called clones of each other. Term Clone is applied for living organisms or structures. Normally clones of organisms can be described as the organism which have the exactly same genetic composition. A clone consists of asexual progeny of a single individual or cell, while the process or technique of producing a clone is called cloning.
In other words, we can say the process of producing genetically similar molecules, cells or organisms from a common parent by asexual reproduction in vitro or in vivo is called cloning. Microbes, plants and some lower animals which reproduce asexually produce natural clones. Twins that develop from same zygote (monozygotic) are also clones.
Cloning can be studied under following headings:
1. Cell cloning
2. Gene cloning
3. Microbial cloning
4. Plant cloning
5. Animal cloning
1. Cell cloning:
The culture of a cell to produce its identical copies is called cell cloning. They are identical genetically, physiologically and morphologically. Only a totipotent can normally produce its clone. Plant cells are totipotent, but animal cells are not totipotent. They are pluripotent (capable of getting transformed into any kind of cells). Therefore, the cloning of plant cells is much easier than animal cell cloning.
2. Gene cloning:
It refers to the production of large population of a DNA fragment. It can be formed simply by application of genetic engineering principles.
3. Microbial cloning:
Microorganisms, such as bacteria, PPLO (Mycoplasma) reproduce by asexual methods. These organisms can be duplicated very easily in culture medium in very short time. Now a days, a genetically altered microbial cell can produce thousand of identical off springs in vitro conditions.
4. Plant cloning:
Cloning of plants is easier because plant cells are totipotent. Rapid production of plant and plantlets can be achieved by meristematic cells, which are usually present in root and shoot apex of plants. If these cells are taken out and cultured in aseptic condition many plantlets can be produced. Plant cloning is very helpful for developing GMF (Genetically Modified Food), drought and pest resistant crops.
5. Animal Cloning:
“Dolly” the world’s first mammalian clone was formed from a completely differentiated non-gametic cell of sheep. It was born on 13th February 1996 at Roslin Institute in Edinburgh (UK). The credit for its birth goes to Dr. Ian Wilmut and his colleagues. Scientists at Scotland cloned the sheep’s Polly and molly.
“Dolly” challenges the fundamental principle of developmental biology. Wilmut and coworkers took cells from the udder of a six years old sheep. Unfertilized egg of another adult sheep was taken out. The egg was de-nucleated. Non dividing nucleus of an udder cell was taken out and inserted into the de-nucleated egg.
In culture medium, the egg began to cleave (divide) forming embryo. The young embryo was then implanted in the uterus of a third sheep (called surrogate mother). The surrogate mother (fertile female sheep) gave birth to a normal healthy lamb, named as “Dolly” (Fig. 1.5).
With the successful cloning of “Dolly”, the possibility of human cloning have been strengthened. Many geneticists all over the world are trying to develop human clones. However, it has been banned by the governments of many countries because of certain social and ethical problems related to it.
Applications of Animal Cloning:
Pig is considered a suitable donor of organs for transplantation to humans. Genetically engineered pigs or suitable breeds of pigs can be cloned for organ transplantation. Population of endangered species of animals can be increased by cloning. Cloning can be of immense use in improving the pedigree of livestock. Superior breeds of animals can be multiplied by this technique.
Applications of Gene Cloning:
(i) Medical Utility:
Bacteria may be used as living factories for synthesizing insulin, growth hormone, interferon’s, vitamins and antibodies by introducing into them the genes which code for these substances along with the plasmids.
(ii) Agricultural Utility:
The nitrogen fixing genes of bacteria may be transferred to the major crops to boost food production without using expensive fertilizers.
(iii) Defective Genes in the Foetuses:
Recombinant DNA technology is useful in knowing— defective genes in the foetuses. Some of these genes can also be repaired.
(iv) Gene Library:
The various clones representing all the genes of an organism are called gene library of that organism. From gene library, a clone having a specific gene can be identified and this gene can be multiplied by growing the relevant clone in a culture for study. The base sequence in this gene can be found. From the base sequence, the sequence of amino acids in a polypeptide can be worked out on the basis of the triplet code.
Gene Library:
A chromosome of an organism is broken into small fragments comprising one or a few genes with the help of restriction endonucleases. Each fragment is joined to a suitable vehicle DNA, forming recombinant DNAs of different nature. These are then introduced into a host (bacterial, yeast, plant or animal) cell.
The foreign DNA fragment replicates as the host cell divides. This produces a group of cells, each containing a foreign DNA fragment comprising one or a few genes. The daughter cells identical to the parent cell are referred to as a clone of cells. The process is called DNA cloning.
Many clones of the cells, each clone containing a different fragment of the same foreign DNA, are thus formed. All the clones are together called gene library because they together represent all the genes of an organism under study. Clones of cells are now tested for identification of the gene of interest through its product.
The gene is multiplied further through cloning. It can be isolated and sequenced to know the arrangement of bases, genetic code and possibly the amino acid sequence in its product. Gene Libraries can also be created using RNA.
The enzyme Reverse Transcriptase, a RNA-directed DNA polymerase, is used to convert the RNA into complementary DNA or cDNA, which can then be converted in the same way as DNA cloning. These gene libraries are maintained through special techniques.
Importance of Gene Library:
Construction of genomic library involves:
1. Isolation of specific gene from different species.
2. Transfer the specific gene to vector DNA.
3. Formation of different types of recombinant DNA.
4. Cell with recombinant DNA is allowed to multiply in culture then it produces clones of cell. The daughter cell carries the same genes as were present in the parent cell.
5. All the clones together are known as gene library.
6. According to the need of the plant, this new gene can be incorporated to produce a transgenic plant.
Gene Bank:
A gene bank is repository (place where things are stored) of clones of known DNA fragments, genes, gene maps, seeds, spores, animal cells, frozen eggs, sperms or embryos, etc. The preservation at very low temperature (say— 196°C, the temperature of liquid nitrogen) is called cryopreservation (deep freeze).
At very low temperature there is little like hood of any change, growth, cell division and mutations because all biological activities essentially cease. Store materials are used in genetic engineering and breeding experiments where species are going to become extinct or to improve the race.
These gene banks will also be used increasingly as there is decrease in the Earth’s biodiversity and genetic variety. As gene banks provide materials for conducting experiments so the genomic information is available for some species. In this aspect, the most important contribution is of Human Genome Project.
Importance of Gene Bank:
1. Gene banks provide information about the structure and organisation of genes present in eukaryotic genome.
2. Construction of gene map of chromosome is possible.
3. It is an ideal method for preserving the genes in bacterial cell.
4. It provides desired DNA for gene manipulation.
5. Some specific gene can be obtained from gene bank for the production of some specific chemical product.
Cybrids/Cytoplasmic Hybrids:
Cybrids are cells or plants containing nucleus of one species but cytoplasm from both the parental species.
Techniques of Cybrids:
Cybrids are produced by the following techniques:
1. Fusion of normal protoplasm of one species with an anucleate protoplast of other species.
2. Fusion of normal protoplast of one species with a protoplast having an inactivated nucleus of other species.
3. Elimination of the nucleus of one species from a normal heterokaryon.
4. Gradual elimination of the chromosomes of one species from a hybrid cell during subsequent mitotic division.
5. By preparing anucleate protoplast (cytoplast) of one species and fusing it with normal protoplast of other species.
Objectives of Cybrid Production:
To combine the cytoplasmic genes of one species with the nuclear and cytoplasmic genes of the other species.
Advantages of Cybrids:
1. Transfer of plasma genes of one species to the nuclear genes of the other species.
2. To prepare sexually incompatible combinations.
3. Recovery of recombinants between the parental mitochondrial or chloroplast-DNA.
4. Production of wide variety of combinations of the parental and recombinant chloroplast with the parental or recombinant mitochondria.
5. Mitochondria from one parental species may be combined with the chloroplast of the other parental species.
Technique # 2. Plant Tissue Culture:
Man is dependent on plants for food, shelter and clothing. It is this dependence of man on plants that has led him to discover different ways to protect and preserve them from being lost to natural and man-made calamities. While doing so, scientists hit upon a technique whereby plants can not only be protected from being lost but are able to develop into a complete plant from a single cell or a small plant part. This technique, called tissue culture, subsequently proved to be a boon for the mankind.
Theoretically, any plant cell—except those lacking a nucleus or enclosed by a rigid, secondary wall—is potentially capable of regenerating the organism (i.e., plant) from which it was taken. Such a cell is said to be totipotent. Collections of similar cells form tissues, and tissue systems are organized into organs, and the specific spatial arrangement of organs constitutes the organism (i.e., full plant).
Plants can be regenerated in vitro (i.e., in laboratory, in a controlled environment) from organ explants (root and shoot tips, buds, leaf primordia, developing embryos, bud scales, and so on), tissue explants (pith, cortex, epidermis, phloem, nucellus), cells (parenchyma, collenchyma, uni-or binucleate pollen-grains) and protoplasts. Figures 1.10, 1.11; illustrate some of the important pathways of tissue culture.
Historical Background of Tissue Culture:
The concept of tissue culture dates back to 1878 when a German Botanist Vochting said that from a small plant piece, a whole plant can be regenerated. Later, other scientists like Haberlandt in 1902 tried to develop in vitro (in controlled environment of laboratory) techniques to demonstrate the totipotency of plant cells. Totipotency is the ability of a plant cell to develop into a complete plant.
Haberlandt reported culture of isolated single palisade cells from leaves in Knop’s salt solution enriched with sucrose. The cells remained alive for up to one month, increased in size, accumulated starch but failed to divide. Efforts to demonstrate totipotency led to the development of techniques for cultivation of plant cells under controlled conditions.
Later, one of his own students, Kotte made a-successful attempt in 1922 in culturing cut root tips of pea and maize in a medium containing animal meat extract called Liebeg’s meat extract which was subsequently replaced by yeast extract and similar results were obtained.
This was followed by an important finding by another scientist named P.R. White of U.S.A. in 1934, who observed an indefinite growth of tomato roots on a specific nutrient medium (which was later called White’s medium) containing vitamins like pyridoxine, thiamine and nicotinic acid. Most of the modern tissue culture media have been derived from the work of Skoog and coworkers during 1950s and 1960s.
Later studies stressed the role of various macro and micro nutrients and their importance in the culture media. Subsequent research findings showed that many more important compounds are necessary for the complete regeneration of a plant body.
These were identified as growth promoting substances and included compounds like auxins such as 2, 4-D (2, 4-dichlorophenoxyacetic acid), cytokinins such as BAP (Benzyl aminopurine), gibberellins etc. When supplied to the culture medium at a particular time point, these compounds would allow the growing cells to differentiate into root, shoot or leaf.
The past few years have witnessed a dramatic increase in our ability to manipulate and study plant cells in culture. Guha and Maheswari (1964) developed the technique of haploid culture. Barski et. al. (1960) performed protoplast fusion in animals, while Carlson et. al. (1972) perfected this technique for plants.
The variety of techniques that collectively comprise “plant cell culture” have permitted investigations at many levels—molecular, cellular, and organismal—and have been applied to a range of disciplines—biochemistry, genetics, physiology anatomy and cell biology. Some of the most important tools in modern genetics have emerged from work with cultured plant cells.
The growing realization of the potentialities of plant cell culture for plant propagation and breeding has itself provided enough impetus for research. Commercial plant propagation using shoot tip cultures is now being implemented, new breeding lines have been selected using protoplast fusion, and new varieties have been developed via somaclonal variation and from plants derived from cultured anthers.
Furthermore, the increasing competence to manipulate plant DNA and genes has to the appreciation that cell culture will be necessary to recover modified plants. All in all, it is now fully realized that cell culture is the keystone to progress in plant biotechnology The application and development of current techniques is opening the door to a second green revolution.
Totipotency:
The ability or capacity of the mature living cells when free from the plant body develop into a new organism in controlled condition is known as Totipotency (L. totus = entire, potential = power). Haberlandt in 1902 (German botanist) has first given the concept of cellular totipotency in plant body.
He stated that ever living cell of the plant body able to regenerate the whole plant body because it is derived from the fertilized egg and contain hereditary information. He tried to grow isolated green cells of leaves but failed to achieve the success.
Later in 1950 EE. Steward got success in proving the cellular totipotency. Cellular totipotency is a property of plant cell because a differentiated plant cell retain its capacity to give rise to a whole plant but in animal cell its losses the capacity of regeneration after differentiation.
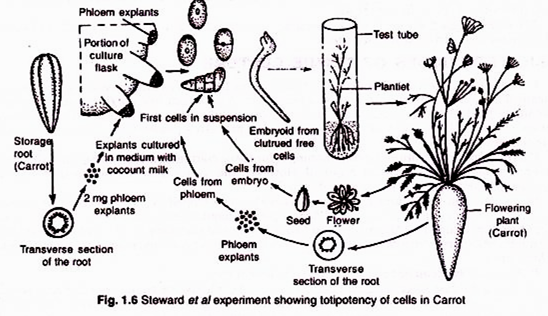
Steward Experiment:
Steward et al took the 2mg. slices of phloem tissue of carrot (Daucus carrota) and grown in a liquid nutrient medium containing coconut water. The part of the plant is taken for tissue culture is known as Explants (Ex. Phloem tissue of Carrot root). The liquid nutrient medium containing explants was allowed some hours for shaking. Then the culture cell divide continuously to form mass of undifferentiated tissue known as callus.
Some of these cell cluster started differentiating the initial of root. When it transfer to semi solid medium then it develop shoot system and give rise new plant. These plant then transfer to pot/soil where they develop in to flowing plant.
Steward experiment shows that in plant even mature (fully differentiated cells) can dedifferentiate, divide, come together re-differentiate and produced a new plant. Plant tissue derived form root, stem, leaf, vegetative bud, floral bud, anther & embryo can exhibit cellular totipotency.
Application:
Totipotency character of plant cell has been used in tissue culture as follows:
1. It helps in rapid multiplication of plant with desirable character.
2. Multiplication of rare plants.
3. To pass the seed dormancy.
4. Quick propagation of useful plants.
5. Develop haploid plant.
6. Produce virus free & disease resistant plant.
7. Help in protoplast fusion & somatic hybridization.
8. Produce high yielding varieties crop plants.
9. Help in embryo rescue.
Various Types of Plant Tissue Culture:
Plant tissue culture includes cell culture, protoplast culture, organ culture, meristem culture etc. (see following table). The organ culture includes any plant organ which has separate identity such as anther culture, ovule culture, embryo culture and bud culture.
The plant part which is used for regeneration in tissue culture is called explant. It may be a cell, a protoplast, a tissue, or an organ. A mass of unorganized regenerated cells in culture medium is called callus (pleural calli) and suspension of free cells of callus in a liquid medium is known as suspension culture. The regeneration capacity or ability of a plant cell to develop into a whole plant is known as totipotency, which reveals that each cell is capable of giving rise to a complete plant.
The cell and tissue cultures lead to regeneration of complete plant. In some species like carrot, and sandalwood, somatic embryos are developed, but in several crops like wheat, rice, barley and tobacco development of both root and shoot takes place from the calli. The entire vegetatively produced descendants of a somatic cell are collectively called clone. An individual member of a clone is called ramet.
Technique # 3. Transgenic (Genetically Modified Organisms):
Genetically engineered organisms are referred to as transgenics or Genetically Modified Organisms (GMO). In other words, a genotype developed by the process of genetic engineering is called transgenic (pleural transgenics). It may be a plant, an animal or microbes such as fungi, bacteria and viruses. Thus transgenic plants or Genetically Modified Plants (GM Plants) are those plants which are developed by the techniques of genetic engineering.
Transgenic plants are obtained involving tissue culture and genetic engineering techniques. The process of transfer, integration and expression of transgene in an organism is called genetic transformation. Transgenes refer to foreign genes or modified genes of the same species which are used for the development of transgenic plants or genotypes. Transgenes maybe from the same species (in modified form), related wild species, unrelated species and microbes (bacteria, fungi and viruses).
Transgenic Breeding and its Advantages:
Genetic improvement of crop plants, domestic animals and useful microorganisms through biotechnology (tissue culture and genetic engineering) in relation to their economic use for mankind is referred to as transgenic breeding.
Transgenic plant breeding refers to genetic improvement of crop plants through the use of transgenes. Now a days, transgenic breeding is being used for specific genetic improvement of different field crops. Transgenic breeding is expected to play an important role in the genetic improvement of field crops in the years ahead.
The main advantages of transgenic crop breeding are briefly discussed below:
1. Rapid Method of Crop Improvement:
Transgenic breeding is a rapid method of crop improvement. By this technique, stable transgenic plants can be developed in 3-4 years, whereas it takes 12-15 years to develop a new variety through conventional methods of breeding such as pedigree, bulk and back cross methods. The first generation of transgenic plants obtained through regeneration is called T0 progeny.
Transgenic plants which are obtained from T0 plants are designated as T1 and those obtained by growing embryos of T1 plants are called T2 plants and so on. In barley and sunflower, transgene exhibited Mendelian pattern of segregation i.e. 3: 1 ratio.
2. Overcome Crossing Barriers:
Transgenic breeding permits gene transfer between unrelated species and even between unrelated organisms. It permits gene transfer even between plants and animals. For example, a freezing resistant gene has been transferred from fish to cultivated tomatoes. Similarly, ovalbumin gene of chicken has been transferred in alfalfa for improving protein quality. There are many other examples of gene transfer between plants and animals.
3. Evolution of New Genotypes:
Sometimes, transgenic breeding may lead to evolution of altogether new plant species, because it permits gene transfer between various plant species. Thus it will affect the process of natural evolution.
4. Application:
Transgenic breeding can be used for the genetic improvement of both autogamous and allogamous crop plants. Both seed propagated and vegetatively propagated species can be improved through the use of transgenes.
5. Effectiveness:
Transgenic breeding has been found effective for the genetic improvement of monogenic characters only. It has not been used, so far, for the genetic improvement of polygenic characters. It has been found very effective in development of plants with resistance to various diseases, insects and herbicides.
Limitations of Transgenic Plant Breeding:
Transgenic breeding has some limitations. The main limitations are:
(1) Unstable performance,
(2) Pleiotropic effect {i.e., multiple effects of a single gene) of transgene,
(3) Position effect of transgene,
(4) Costly method of crop improvement,
(5) Low frequency of transgenic plants,
(6) Risk of evolution of problematic weed Species, etc.
Moreover, polygenic characters cannot be manipulated by genetic engineering.
Criticism Against Genetically Modified Crops:
(i) Unintended harm to other organisms:
A laboratory study, published in ‘Nature’, reported that pollen from BT corn caused high mortality in monarch butterfly caterpillars. Monarch caterpillars consume milkweed plants, not corn, but the fear is that if pollen from BT corn is blown by the wind onto milkweed plants in neighboring fields, the caterpillars could eat the pollen and perish. Although the ‘Nature’ study was not conducted under natural field conditions, the results seemed to support this viewpoint.
(ii) Reduced effectiveness of pesticides:
Just as some populations of mosquitoes have developed resistance to the now-banned pesticide DDT, many people are concerned that insects will become resistant to BT or other crops that have been genetically modified to produce their own pesticides.
(iii) Gene transfer to non-target species:
Another concern is that crop plants engineered for herbicide tolerance and weeds will cross-breed, resulting in the transfer of the herbicide resistance genes from the crops into the weeds. These “super weeds” would then be herbicide tolerant as well. Other introduced genes may cross over into non-modified crops planted next to GM crops.
Genetically Modified Food:
The food prepared from the produce of genetically modified (= transgenic) crops is called genetically modified food (= GM food).
GM food differs from the food prepared from the produce of conventionally developed varieties in the following aspects:
(i) It contains the protein produced by the transgene in question, e.g., Cry protein in the case of insect resistant varieties.
(ii) It contains the enzyme produced by the antibiotic resistance gene that was used during gene transfer by genetic engineering.
(iii) It contains the antibiotic resistance gene itself.
Disadvantages of GM Food:
GM food can lead to the following problems:
1. Allergies:
The transgenic food may cause toxicity and or produce allergies. The enzyme produced by the antibiotic resistance gene can cause allergies, because it is a foreign protein.
2. Effect on Bacteria of Alimentary canal:
The bacteria present in the human alimentary canal can take up the antibiotic resistance gene that is present in the GM food. These bacteria may become resistant to the concerned antibiotic and will be difficult to manage.
3. Economic concerns:
Bringing a GM food to market is a lengthy and costly process, and of course agri-biotech companies wish to ensure a profitable return on their investment.
Transgenic Animals:
The animals which carry foreign genes are called transgenic animals.
Production of Transgenic Animals:
The foreign genes are inserted into the genome of the animal using recombinant DNA technology.
The production of transgenic animals includes:
(i) Location, identification and separation of desired gene,
(ii) Selection of proper vector (generally a virus) or direct transmission,
(iii) Combining the desired gene with the vector,
(iv) Introduction of transferred vector in cells, tissues, embryo or mature individual,
(v) Demonstration of integration and expression of foreign gene in transgenic tissue or animal.
Why are Transgenic Animals Produced?
It is aimed at;
(i) Production of pharmaceutically important drugs and proteins in large quantity in cows, sheep and goats,
(ii) To grow spare organs (e.g., heart, pancreas) of pigs for human use (xenotransplantation),
(iii) Studying various aspects of cloning and effect of biochemical in mice,
(iv) Producing human cell lines for curing genetic disorders, e.g., Alzheimer, hemophilia, thalassemia, etc.
(v) Replace defective parts with freshly grown part from patients own cells,
(vi) To produce human clones if ethics allow the same.
Examples of Transgenic Animals:
Some important examples of transgenic animals are given in the following table: