ADVERTISEMENTS:
A. Thermal Stress:
1. Forewords:
A large amount of studies have been carried out in which stress to cells was provided in the form of heat shock, i.e., exposing the cells to elevated temperatures (40-42°C, in some cases up to 60°C as well).
It is a known fact that prokaryotes possess only a single copy of a heat shock gene.
This fact, coupled with the essential function of most heat shock genes, necessitates the constitutive expression under all conditions of most prokaryotes regulatory stress genes. However in general, eukaryotes possess at least two copies of heat shock genes. Of the two, one is under heat shock regulation and the other is under constitutive control.
2. Stress gene inductive regulation and control mechanism:
ADVERTISEMENTS:
In prokaryotic cells the heat shock regulon is defined by the rpoH gene. Its product is a 32 KD sigma factor (σ). For instance, it has been reported that the major heat shock genes of E. coli are under the control of the rpoH (htpR) gene which codes for σ heat shock factor. The σ factor directs core RNA polymerase enzyme (Eσ) to recognize the promoters for heat shock genes.
The studies on how the cell uses σ to regulate the heat shock response have provided a great deal of information about the molecular mechanisms involved in mounting a transient, global response to environmental stimuli. The evidence that in E. coli RNA polymerase containing σ (Eσ) is responsible for nearly all the transcription of the heat shock genes has been shown. It indicates that HSPs are required for cell growth at most temperatures.
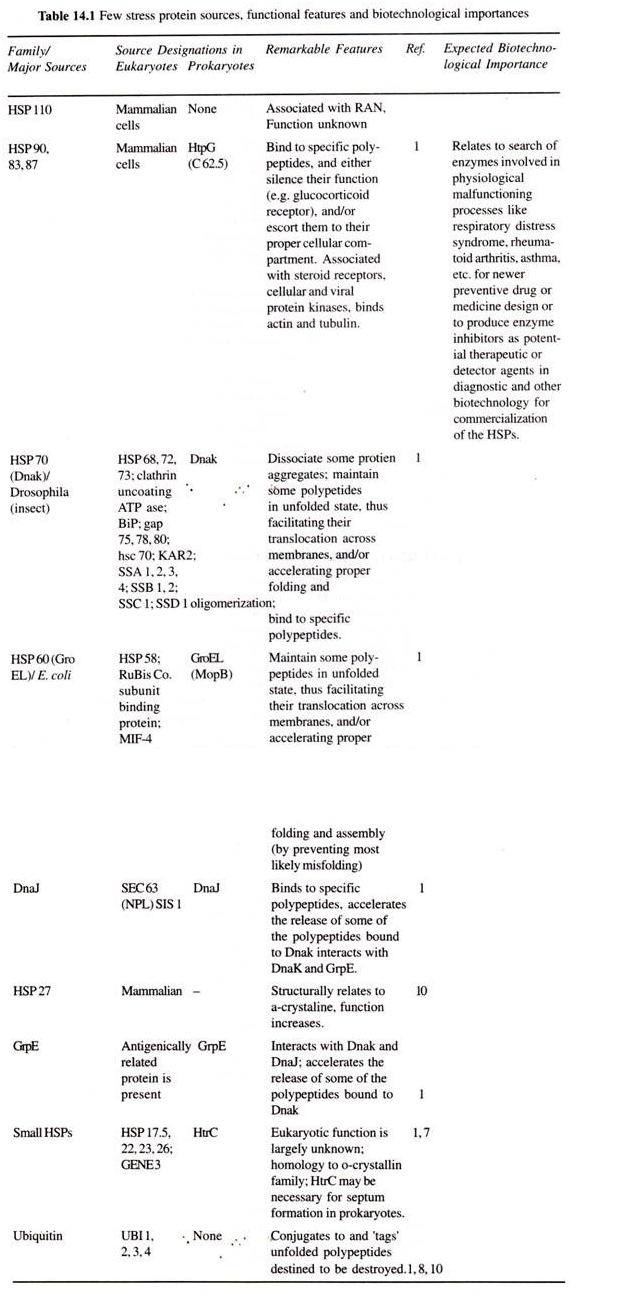
So, the HSPs are involved in thermotolerence and in turn regulation of the heat shock response for the transcription and transition of rpoH mRNA. It also integrates the stability of σ and is regulated to provide the amount of σ required for heat shock gene expression in stress protein synthesis. As shown in Table 14.1 the HSPs poses diverse functions at the molecular level ranging from the major E. coli σ factor σ (the rpoD gene product) to a cryptic form of lysyl tRNA synthetize (the lys U gene product).
It has been reported that abnormal protein synthesis under adverse conditions, like in sporulation in prokaryotic cells, is accompanied by the appearance of new RNA polymerase specificity factors that read the gene necessary for stress protein formation. It is also stated that the overall structure of RNA polymerase in B. subtitles is essentially identical to that in E. coli (σ2 β σ).
ADVERTISEMENTS:
However, the precise molecular weights of the various subunits differ between gram positive and gram negative bacteria. Vegetative growing Bacillus subtitles’ has been stated to contain multiple factors, namely σ55, Qs37, σ32 and σ28 with molecular weights 55, 37, 32, and 28 KD, respectively.
Each of them can bind interchangeably to the core RNA polymerase (σ2 β β1) This can confer upon it the ability to recognize a different set of promoter sequences. Factors σ and σ seem to be involved in switching on genes that are expressed at the very onset of sporulating protein synthesis under stressed environmental condition(s).
In exhaustion of nutritional components like carbon, nitrogen, or phosphorous, at least two new sporulation protein specific factors have been stated to be synthesized. One being σ and the other is probably σ that dissociates σ 55 factor from the core RNA polymerase.
In this way σ replaces σ on core polymerase and permits the transcription of the sporulation protein specific promoter to start functioning. Interestingly, the a factors determining the expression of specialized sets of genes are appearing as general features of many prokaryotes as diverse as E. coli, Bacillus and Streptomyces species.
For example, the expression of heat shock stress genes and nitrogen- regulated genes in E. coli is known to be dictated by specific a factors encoding by genes htpR and ntrA. Also, multiple a factors are a feature of most complex bacterial viruses such as T4, λ, T7, μ, and B. subtilis phage SPOl.
More recently, it has been noticed that the rpoH (σ) gene of E. coli can be deleted as long as its culture has been maintained below 20°C. If this culture is however, shifted to high temperatures, its level of σ changes in various ways. Firstly, the rpoH mRNA is transiently stabilized; secondly, the rpoh gene transcription is stimulated; and thirdly; the half life of σ is transiently increased. Because of the very short half life of σ polypeptide (45 sees) the intracellular level of σ immediately causes variations in the parameters controlling σ production and stability.
Thus, it is important to know how and which proteins play a role in stress alterations like mutations allowing cell growth at all temperatures to take place. It relates to an organism response at the cellular and molecular level when confronted with sudden changes in environment and molecular adaptation in the ability of the cells to acclimate themselves to the new environment.
3. Adaptive expression:
Although much information has not been accumulated on this, some light has been thrown on the E. coli cell system. In this organism three interesting twists in rpoH gene expression have been reported. In the first twist, rpoH is expressed from at least four different promoters.
ADVERTISEMENTS:
In the second twist, two of the promoters are negatively regulated by the DnaA protein (required for inhibition of E. coli DNA replication as well as global regulation). The third promoter is under CAP-CAMP catabolite control, and the fourth is under the control of a more recently known σ24 (σE) factor. The gene coding for σ has not been identified as yet, it is, however, clears that σ 24-promoted transcription is accelerated at high temperature of 50°C range. It indicated that there are at least two heat shock systems in E. coli.
The conventional one is regulated by σ32, and more currently known under σ24 regulation. Heat shock genes at high temperatures like 50°C are transcribed exclusively by either Eσ32 or Eσ24, and Eσ24 levels, are continuously replenished by the σ24 -promoted transcription of the rpoH gene.
So, it is stated that heat shock gene HtrA (degP) is under the exclusive transcriptional control of Eσ24. Its product is essential for viability of E. coli at higher temperatures and remains under heat shock adaptive regulation. It entells that these two heat shock regulons are interconnected. The third twist promises rpoHmRNA translation under negative repression control as well. Signals affecting repressor element activity alters intracellular σ32 levels.
It appears that adaptive expression of stress has been known for a long time; however, its actual application in biological and physiological systems has been recognized only recently. Adaptive expressions in (a) ischemic stress associated with lethal ischemic injury, (b) pathogenesis in stress-related diseases. (c) modifying microenvironment of the lipid bilayer and modulating involved enzyme activities. etc., have recently also been known.
ADVERTISEMENTS:
Participation of stress proteins like HSP70 and HSP68 in cellular adaptive functional expressions has also been shown. Such an adaptation expression process usually takes a long time until the cellular components of living organisms possessing intrinsic molecular mechanism adapt to the stressful situation.
The actual molecular adaptive expression, although, is a long term process; many changes can still occur during the initial phase of adaptation. They provide adjustment of cells to the stressful situation and temporarily maintain them in the new environment.
However, report is also available regarding short-term adaptive expression in prokaryotic cells to stress conditions. It appears; therefore that adaptive expression depends largely on the source cell as well as the stressor type. For example, the results of a recent study on vascular endothelial cells (VEC) indicated that VEC promptly responded to the stressor if it is in the form of oxidative stress.
This prompt response was manifested by synthesizing several oxidative stress proteins including HSP and stimulating antioxidative enzymes. Apparently, it was to protect the bio-systems from the toxic effects of stress allowing them to recover and survive.
B. Oxidative Stress:
1. Forewords:
It has been indicated that the constitutive restriction enzyme Bam H1 protein biosynthesis in Bacillus amyloliquefaciens H1 strain, is greatly influenced by oxygen tension in the cell cultivation liquid. The profiles of Bam HI production, as shown in Figs. 14.5-14.7, show that specific growth rate (µ) and specific restriction enzyme protein yield (YRE/X) of this prokaryotic cell varied largely, but YRE/X to only a little extent under thermal processing stress.
ADVERTISEMENTS:
The variations of these bioprocess technology state parameters by oxygenation and shear control parameter were seen to be very pronounced. It appeared that formation of Bam H1 involved reactive oxygen species stress gene(s) having functional optimality at specific oxygen tension. In a prokaryotic system, oxidative stress has been found to induce heat shock proteins and glucose regulated proteins.
This led the scientists to believe that such cellular response in eukaryotic cells may be of great significance, since reactive oxygen species have been shown to play a significant role in a variety of disease processes like heart attack, stroke, arthritis and a few others. Reactive oxygen species have also been implicated in ischemic and reperfusion injury to a large variety of mammalian tissues.
However, information on the prompt response of eukaryotic cells to the oxidative stress is scanty. Prompt molecular adaptation of vascular endothelial cells to oxidative stress as mentioned earlier is an important piece of information. In Bam HI producing prokaryotic cells, the nature of the oxygen species reactive stress genes and corresponding stress factors in the cell are still not identified.
Their identification and involvement in thermotolerence and oxygen stress in Bam H1 yield increase (Figs. 14.5 & 14.6) remains as a future research scope in the area of oxidative stress in prokaryotes. It may also be necessary to know in this organism whether the unknown individual HSPs are under the control of constitutive or inducible promoters.
Oxidative stress developed by exposing bacteria (Salmonella typhimurium) to low concentration of H2O2 induced several new proteins including HSPs and mRNA of catalase. In the process of oxidative stress adaptation, the bacteria become resistant to killing by otherwise lethal doses of H2O2 and other strong oxidants.
Thus, it may appear in mammalian cells that oxidative stress induction creates a defence action against subsequent cellular injury by expressing a distinct group of proteins such as HSP and GRP, etc. This class of special proteins has been collectively termed as ‘oxidative stress inducible proteins’ (OSIPs).
Furthermore, it was shown that among the oxidative stress inducible genes, ROXY gene positive regulatory gene for at least 9 different proteins including catalase, hydroperoxide I, glutathione reductase, and alkyl- hydro peroxide reductase. However, many oxidative stress-inducible proteins are not characterized; and their functions are yet unknown.
2. Possible molecular mechanisms:
ADVERTISEMENTS:
From the influence of dissolved oxygen on constitutive enzyme protein Bam H1 formation by Bacillus amyloliquefaciens H1 strain as well as prokaryotic systems like S. typhimurium and others under oxidative stress, it will be interesting and useful to know about molecular mechanism of the participation of oxygen in such situations. The ambivalent mechanism of oxygen in aerobic organisms is really a paradox.
A beneficial effect of oxygen in aerobic organisms up to a certain level has been observed. Above the critical level an adverse effect was observed in many cases including Bam H1 production. It tends to suggest that below a certain level (KLa < 260h-1) oxygen does not activate the regulon of mRNA of the activator protein to react with its oxyR binding site which is responsible for changing the oxidation state.
It permits in vivo Bam HI synthesis to continue and increase. On the other hand, above a certain dissolved oxygen level; i.e. for the volumetric oxygen transfer coefficient (KL a) > 260h-1, it activates the oxyR binding site of the activator protein. The oxyR protein is, therefore, directly activated by metabolic oxygen stimulus, an oxidant becoming a transcriptional inactivatory by generating reactive oxygen species. It is generated from high dissolved oxygen in the medium and interacts with oxyR changing the oxidation state of its binding site. This change perhaps causes conformational change that affects the way in which the proteins interact with their corresponding promoters.
So, the oxyR regulon response to high dissolved oxygen stress is one of the few molecular mechanisms for which the translation of an environmental stress into transcriptional control has been defined. Some cells were partly resistant to this response to survive harmful metabolic effects of active oxygen species and possibly other oxidants in the medium.
In both eukaryotic and prokaryotic bio-systems, molecular oxygen may, therefore, play an ambivalent role. A substantial oxidative stress damage rate to DNA can occur as a part of normal metabolism and of lipid peroxidation in cells. The oxidative DNA damage rate has been measured by thymidine glycol excretion in mammals. In order to cope with oxidative stress, aerobic organisms evolved enzymic and nonenzymic molecular level antioxidant defense mechanisms.
These molecular mechanisms within the organisms have been evolved to limit the levels of reactive oxidants and the damage they caused. In cell proliferation the molecular mechanism of oxygen stress dependent pathway has been shown to play a significant role by regulating uridine phosphate mediated ribo-nucleotide reductase system.
ADVERTISEMENTS:
In a most recent study, it was demonstrated that IL-1 can induce SOD activity and also able to express heat shock proteins. IL-1 α is also a prominent member of a group of polypeptide mediator called cytokines. IL-1 α has recently been found to function as a therapeutic agent when used at low doses. It was also demonstrated recently that IL-1 α reduced the myocardial ischemia/reperfusion injury when treated with this cytokine for 48 hours. IL-1 α also induces the expression of the mRNA for HSP27 within four hours of treatment.
From various literature information’s and the above discussions, the manifestation of molecular mechanisms of oxidative stress possibly may be of the following categories:
a. Inductive oxidative damage and protection (restriction of cell proliferation).
b. Antioxidant protection (by scavenging mechanism).
c. Enzymic control (possibly by gene regulation, e.g., in SPS formation).
d. SOD regulation and control (molecular adaptive mechanism).
e. Metabolic oxygen control coefficient regulation (possibly by induction-repression mechanism of conformational protection, e. g., in restriction endonuclease formation).
f. Therapeutic regulation (possibly delayed molecular adaptation mechanism, e. g., in ischemic heart).
C. Other Stresses:
Cold shock (thermal) and photon stress are shown to be important in transitional stress protein development in phases of some of the plants cell cycle affecting synthetic and organelle activity. These plants growing at subnormal or cold temperatures and under low photonic intensity may have large nucleoli with higher amounts of cellular RNA and increased RNA polymerase activity. Growth of seedlings of Triptycene aestivum cv Chinese Spring in darkness between 5°C to 20°C has been cited as an illustrative example.
The maximum growth rate increased from 0.6 mgh-1 at 5°C to 3.1 mgh-1 at 20°C and maintaining this value at 25°C is an indication that the cell cycle would be expected to be longer compared to that at 20° C. So, cells compensate by loosing RNA levels and presumably stress proteins in order to sustain a minimal amount of growth.
It is also indicative that cold shock may block the cell cycle and cold shock induced quiescent cells may be different from those of the normal in terms of deficiency of cell cycle related proteins. Generation of stress proteins under cold shock might have prevented the plants from entering into S phase from G1 phase. It may, therefore, appear that in cold perennial plants remain quiescent at temperatures below 5°C accumulating abnormal stress proteins for sustenance until the next favorable climate.
A direct usefulness of this may relate to plant biotechnology in having plants remain in quiescent state during long periods of cold and rapidly useful to exploit transient periods of elevated temperatures or rainfall. It will, however, depend on the adaptive expression response of the plant cells. Cold stress proteins, thus, bear important concerns in daily life to infantry in cold regions, marine operations, cryobioepisodes, cold injury, preserved foods, and many others.
Until today, however, very little evidence has been shown for induction of transcription of any identifiable gene by cold shock treatment either prolonged or of rapid ‘cold shock’ type. It, therefore, remains as a big challenge and scope for genetic and metabolic engineers. Possibilities of generations of stress proteins under mechanochemical, electrochemical, and other stresses (Fig. 14.8) do exist in the contraction of skeletal muscle. Two membrane-bound stress proteins, 42 KD and 24 KD, have been reported to be expressed with iron limitation in Bacteroides gingivalis at 37°C.
It appears, therefore, that cellular adaptation to a variety of external stresses is essential for the survival of both prokaryotes and eukaryotes. However, unlike HSPs, the majority of cold shock proteins are of relatively low molecular weight. For example in response to cold temperature, E. coli rapidly induces several polypeptides including Nus A, Rec A, dihydrolipoamide, acetyl transferase subunit of pyruvate dehydrogenase, polynucleotide phosphorylase, pyruvate dehydrogenase as well as some initiation factors.
One of these cold shock proteins, F 10.6 (10KDa), is synthesized only during growth at low temperature. Some of these proteins (viz., Nus A) are likely to be involved in transcription and translation as well as mRNA degradation. Recently a 7.4 KDa protein (CS 7.4) has been found to be induced when E. coli culture temperature was shifted from 37°C to 10-15°C.
It has been possible to clone the gene for CS 7.4 (csp A) to determine its DNA sequence and to show evidence that CS 7.4 may play a role in protecting cells from cold injury and, thus, may function as an ‘anti-freeze protein’. Such anti-freeze proteins (but distinct from CS 7.4) are found at high concentration in the serum of polar-dwelling marine fishes and in the hemolymph of the insects that survive in subfreezing climates. It is known that hypothermia restricts bacterial growth by blocking an early step in protein synthesis. The synthesis of these proteins in response to cold seems to be adaptive in nature.
One of the key questions in the cellular adaptation concerns the molecular mechanisms of differential gene regulation. It is quite possible that cold shock can trigger a cascade of second messengers which can simultaneously induce and inactivate specific sets of genes. The gene encoding cold shock protein has been cloned and characterized.
A number of genes induced by cold shock have been identified by a differential hybridization screen of a yeast genomic library. Interestingly, genes for a developmentally regulated membrane protein and for ubiquitin have been shown to be induced in response to both cold shock and heat shock.
Induction of the expression of cold shock proteins has also been demonstrated in eukaryotes. For example, both low and high molecular weight proteins were found to be expressed in the rabbit leg skeletal muscle when the limb was subjected to repeated stress induced by hypothermia (Fig. 14.9). Interestingly, some of these proteins disappeared during subsequent cooling and rewarming (Table14.3).
In a study, the leg of a rabbit was repeatedly cooled by ice, followed by rewarming after each cooling episode. The process was repeated ten times. Such a repeated cooling and rewarming process enabled the leg muscle to withstand the subsequent lethal cold injury significantly better when compared with a normal leg, suggesting that the leg muscle had been adapted to cold by repeated cooling and rewarming.
Not only did this process of adaptation make the tissue less susceptible to cold injury, but it was also associated with the reduction of reperfusion injury. It seems reasonable to speculate that the induction of cold shock proteins play a role in the protective mechanism. Recently the major CSP from Bacillus subtilis (CSPB) has been over-expressed in recombinant E. coli cells. The recombinant cell was developed by using the bacteriophage T7 RNA polymerase/promoter system.