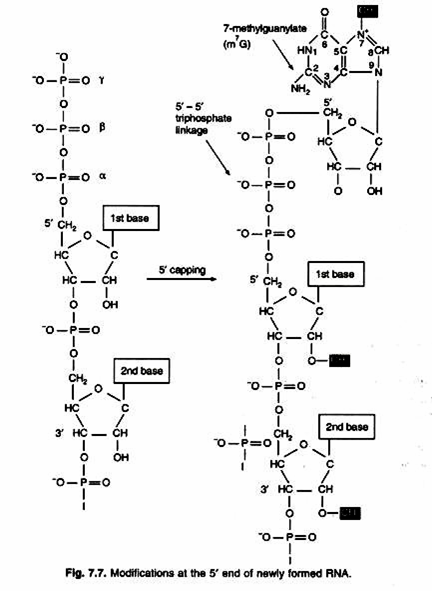
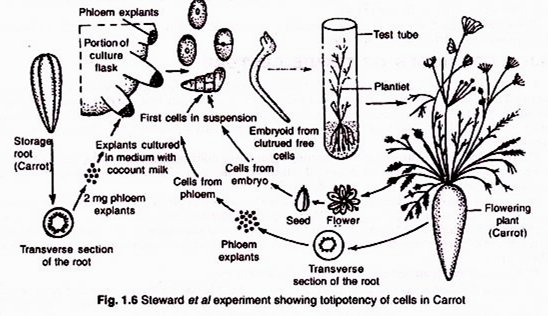
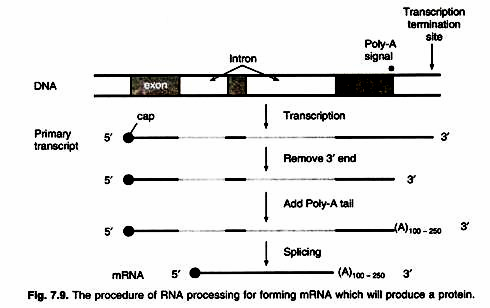
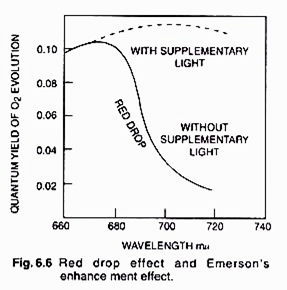
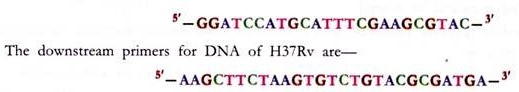ADVERTISEMENTS:
Substrates/Resources:
1. Molasses Refining:
Molasses is the most common renewable carbohydrate waste resource used for fermentative production of ethanol, baker’s yeast and many other bio-products.
Molasses is the syrup that is left after the recovery of crystalline sugar from the concentrated juice of sugar cane or beet.
Depending on the juice resource and its processing, two kinds of molasses are produced by sugar industries. They are ‘blackstrap’ and ‘hightest’ molasses. Blackstrap molasses has been used principally in industrial alcohol production in distilleries. It usually contains 50-55% of sugars mainly sucrose. However, hightest molasses has been used for ethanol manufacture.
ADVERTISEMENTS:
It is an evaporated juice but most of it remains in inverted sugar form as a result of acid hydrolysis. This type of molasses is usually high in sugar content. Occasionally it may contain as high as 78% sugar. In India and some other countries, blackstrap molassess has been widely used for industrial fermentative production of ethanol.
The industrial process adapted by distilleries usually consists of fermenting the diluted molasses with yeast. In many European countries, from molasses another material is produced by its concentration which is known as “Vinasses” (latin: vinaceus). Vinasses are a product from molassess through its transformation into a valuable, concentrated and marketable by – product.
Vinasses have molasses like properties. An earlier estimate of vinasses production in West Europe as reported in literature is about 6.8 x 105 tons per year based on 65% dry matter. This vinasses has been termed as partly or fully de-sugared cane or beet molasses. It is also produced by desugaring process by the aid of a special method.
Thus, it is clear that molasses is an industrial sugar post-treatment process by – product/residue. Its fermentable sugar serves as substrate/carbon source for a large number of microbes in producing different products by industrial microbial fermentation processes.
ADVERTISEMENTS:
Molasses pretreatment/refining: In using molasses in fermentation industry in many cases for its refining, molasses is heat treated, clarified and diluted before its use. It is usually adjusted to a pH of 4.5 to 5. Next heated nearly to its boiling point. If in fermentation medium CSL (corn sleep liquor) is used, this is added to the molasses before heat treatment. Purpose of heating is to destroy a large percentage of microorganisms present and to aid in the clarification process which follows:
Also, in using molasses in fermentation industry its fermentable sugar/sucrose fraction in many cases needed to be separated from its non-sugar/sucrose salt fraction or in other words, pretreatment/refining was required.
Flow sheets of molasses desugarization and desugared molasses concentration are shown in Figs. 17.1 and 17.2 respectively. This was primarily to supply carbon source in purer form by the sugars contained in molasses. In ethanol fermentation from molasses, it is well known that product yield depends upon molasses origin, i.e. molasses quality.
Likewise, baker’s yeast biomass production from molasses depends on its quality. The relation between degree of treatment/refining on yield or ethanol was reported as in Figs. 17.3, 17.4. In some countries, the cane juice clarification process to produce white sugar uses lime and sulfite. Fig. 17.5 shows that an inverse correlation exists between polarization (Pol) and reducing sugar in molasses. It can also be seen from this figure that the more the sulfur that is burnt for molasses, the less is the polarization.
Sulfite decreases the pH, and more reducing sugar/sucrose might be hydrolysed in the process. In Fig. 17.4, it can be seen that there is a negative correlation between reducing sugar in molasses and ethanol yield. By such pretreatment/refining, the ratio of sucrose/reducing sugar could also serve as an indicator of the capacity of molasses to produce ethyl alcohol.
The higher the ratio, the higher is the ethanol produced by fermentation, based on total reducing sugars. The pretreatment for purity of molasses also affects the ethanol yield (Fig. 17.4). This facet agrees with the data shown in Fig. 17.8 because the higher the reducing sugar, the lower is the purity.
ADVERTISEMENTS:
In more recent years, it is reported that molasses contains (1) non-conducting sucrose fraction and (2) non-sucrose salt fraction. In recovering these fractions and for abating pollution in environment, refining/pretreatment of molasses has been carried out by industrial chromatographic separation method.
The efficiency of chromatographic separation of these two fractions relates strongly to the mean particle/bead size of the stationary phase. Report on the effect of mean particle size for molasses purification/separation using 5.5% cross-linked strong action exchange resins (Finex ftd, Finland) in mono-valent form indicated molasses particle size ranged from 100- 500 pm.
Used resin beads were of mono-bead type (i.e. having narrow particle size distribution). Pulse test analysis technique with water as eluant for test mixture was used in evaluation of chromatography separation for refining. One ml fraction from the effluent stream was used to analyse by HP 11OO HPLC system with Na+ column.
In refining by chromatography the performance was characterized (Fig 17.5 to 17.13). For this, relevant column efficiency and moment analysis parameters were used. The pulse response was expressed in terms of first absolute moment as given in equation 17.1. From this the second central moment of the pulse response defined as and given in equation 17.2. As method of moment is valid for gaussian profiles, non-gaussian profiles peak was analysed with caution in interpretation.
The first and second moments of the peaks were applied for the calculation of bed porosity, adsorption equilibrium constant, separation factor, resolution and height equivalent to theoretical plate (HETP). Chromatographic parameters as defined by equations 17.3 through 17.7 were used in analysing data of the experimental set up system characteristics.
It is clear from the foregoing sections that customizing the mean particle size of the fractionation resin to the process allowable minimum is an effective tool for improving cost efficiency of chromatographic refining/separation processes.
For sucrose separation from synthetic molasses at the conditions in the report (0.5m/h), the bead size reduction from 328 µm to 250 µm results in:
i. Concentration increase for sucrose: 24%
ii. Concentration increase for betaine: 16%
iii. Salt peak purity increase: 13%
iv. Sucrose peak purity increase: 7%
ADVERTISEMENTS:
v. Betaine peak purity increase: 13%
Trace element/Mineral refining from Process Biotechnology Medium
Used Methods:
1. Adsorption
2. Chelation
1. Adsorption:
Solution of the medium is mixed with an adsorbent, shakened thoroughly and allowed to settle.
2. Lignocellulose Refining/Pretreatment:
Over the last several decades, scientists and technologists have pretreated lignocellulosic materials for its breakdown products utilization as alternate renewable resource material in process biotechnology. Treatment/refining of lignocelluloses have been carried out both by chemical processing and by bio-processing with the same objective of “biomass refining”.
The main components of lignocellulosics (cellulose, hemicelluloses and lignin) can be fractionated by sequential treatment to produce fraction that may be used for different product applications. One of the important feedstocks of such a category which is abundantly available and renewable is rice husks/hulls and straw.
Refinining or pretreatment of such feedstocks by hydrolysis has been practiced for a long time. Catalytic agents which have been used for the hydrolysis are acid or enzyme. A comparison of activities of acid and enzyme catalysts on three cellulosic substrates at 50°C showed that 100,000 times as much acid is required to bring about the same degree of saccharification of fermentable sugars.
Enzymatic saccharification of paddy hulls and bagasse has been reported. A possible process for the full utilization of biomass may start with a hydrolytic stage (carried out in aqueous media with possible addition of acids) in which hemicellulosic fraction is solubilized, while both cellulose and lignin remain in solid phase with little alteration.
The liquors from the treatment (containing hemicellulose degradation products such as sugar oligomers, monosaccharides, acetic acid, furfural and hydroxymethyl furfural) are easily separated from the solid phase, which can be subjected to further processing (for example, organosolvent pulping) to achieve separation of cellulose and lignin.
Refining of lignocellulosics (carbohydrate composition as in Table 17.5) thus can give its purified component which in turn, can be subjected to further modifications by alkali, solvent and biological/enzymic treatments as described and mentioned in literatures.
In more recent years, rice husk autohydrolytic refining under non- isothermal conditions using hydrothermal acid processing provided a sound basis for the kinetic interpretation of a heterogeneous system where simultaneous degradation reactions occur. It leads to several products of commercial/industrial interest as well as for research and development concerns (Table 17.5).
It has been reported that under the most suitable reaction condition usually employed for autohydrolytic breakdown process, sugar oligomers (mainly xylooligomers) are major breakdown products.
The concentrations of the breakdown products can account for more than 50% of the initial hemicelluloses. Industrially/commercially, xylooligomers are currently used as new sweeteners or food additives. In relation to human health, it has been reported that xylooligomers selectively enhance the growth of Bifidobacteria, thus promoting a favourable intestinal environment performance.
Moreover, the xylooligomer solution/liquids produced in autohydrolysis can be transformed into xylose solutions by acid catalysed post hydrolysis. Availability of xylose solution by performing successive stages of autohydrolysis and post hydrolysis shows advantages for further process biotechnological conversion over the conventional single stage prehydrolysis. This is because the milder reaction conditions result in decreased content of side products causing inhibition of the microbial metabolism.
On treating/refining various types of lignocelluloses which contained cellulose, lignin, protein and ash as given in Table 17.6 encouraging results could be seen. In untreated and treated agroresidues and grass as seen from table, it is evident that different components of agroresidues and grass chauged a lot after refining. In case of alkali treated bagasse and rice straw, the cellulose content increased by 56 and 72 percent with a decrease in lignin content of 47 and 60 percent respectively. But in case of the butanol treatment, the percentage increase of cellulose content was less compared to the alkali-treated substrates.
In equation (17.20), 0H is the percentage of initial xylan converted into high molecular weight xylo-oligomers and OL is the percentage of initial xylan converted into low molecular weight xylo-oligomers. In equations 17.9 to 17.20, koF, ks, ko2H, ko2L, ko3, EoS, Ea2H, Fa2L, Ea3 and EaA denote activation energies and k2max and β give the values of kinetic coefficient and a function of time (τ), while α is a measure of susceptible fraction of xylose in initial xylan. These treatments/ refining analysis of lignocellulosic biomass indicate the possibility of full utilization of the feedstock/ substrate in industrial process biotechnology.
Nomenclature:
c solution phase concentration (g/l)
ADVERTISEMENTS:
HETP height equivalent of the theoretical plate (cm)
K adsorption equilibrium constant
RA/B resolution between components A and B
t time (min)
tr mean retention time(min)
to injection time (min)
u superficial velocity (cm/min)
V volume (I)
z bed height (cm)
Greek Letters:
αA/B separation factor of component A over B
ε bed porosity
μ1 first absolute moment (min)
μ2 second central moment (min2)
σ2 peak variance (min2).