ADVERTISEMENTS:
Germplasm Conservation:
Germplasm broadly refers to the hereditary material (total content of genes) transmitted to the offspring through germ cells.
Germplasm provides the raw material for the breeder to develop various crops. Thus, conservation of germplasm assumes significance in all breeding programmes.
As the primitive man learnt about the utility of plants for food and shelter, he cultivated the habit of saving selected seeds or vegetative propagules from one season to the next one. In other words, this may be regarded as primitive but conventional germplasm preservation and management, which is highly valuable in breeding programmes.
ADVERTISEMENTS:
The very objective of germplasm conservation (or storage) is to preserve the genetic diversity of a particular plant or genetic stock for its use at any time in future. In recent years, many new plant species with desired and improved characteristics have started replacing the primitive and conventionally used agricultural plants. It is important to conserve the endangered plants or else some of the valuable genetic traits present in the primitive plants may be lost.
A global body namely International Board of Plant Genetic Resources (IBPGR) has been established for germplasm conservation. Its main objective is to provide necessary support for collection, conservation and utilization of plant genetic resources throughout the world.
There are two approaches for germplasm conservation of plant genetic materials:
1. In-situ conservation
ADVERTISEMENTS:
2. Ex-situ conservation
1. In-Situ Conservation:
The conservation of germplasm in their natural environment by establishing biosphere reserves (or national parks/gene sanctuaries) is regarded as in-situ conservation. This approach is particularly useful for preservation of land plants in a near natural habitat along with several wild relatives with genetic diversity. The in-situ conservation is considered as a high priority germplasm preservation programme.
The major limitations of in-situ conservation are listed below:
i. The risk of losing germplasm due to environmental hazards
ii. The cost of maintenance of a large number of genotypes is very high.
2. Ex-Situ Conservation:
Ex-situ conservation is the chief method for the preservation of germplasm obtained from cultivated and wild plant materials. The genetic materials in the form of seeds or from in vitro cultures (plant cells, tissues or organs) can be preserved as gene banks for long term storage under suitable conditions. For successful establishment of gene banks, adequate knowledge of genetic structure of plant populations, and the techniques involved in sampling, regeneration, maintenance of gene pools etc. are essential.
Germplasm conservation in the form of seeds:
Usually, seeds are the most common and convenient materials to conserve plant germplasm. This is because many plants are propagated through seeds, and seeds occupy relatively small space. Further, seeds can be easily transported to various places.
There are however, certain limitations in the conservation of seeds:
ADVERTISEMENTS:
i. Viability of seeds is reduced or lost with passage of time.
ii. Seeds are susceptible to insect or pathogen attack, often leading to their destruction.
iii. This approach is exclusively confined to seed propagating plants, and therefore it is of no use for vegetatively propagated plants e.g. potato, Ipomoea, Dioscorea.
iv. It is difficult to maintain clones through seed conservation.
ADVERTISEMENTS:
Certain seeds are heterogeneous and therefore, are not suitable for true genotype maintenance.
In vitro methods for germplasm conservation:
In vitro methods employing shoots, meristems and embryos are ideally suited for the conservation of germplasm of vegetatively propagated plants. The plants with recalcitrant seeds and genetically engineered materials can also be preserved by this in vitro approach.
There are several advantages associated with in vitro germplasm conservation:
ADVERTISEMENTS:
i. Large quantities of materials can be preserved in small space.
ii. The germplasm preserved can be maintained in an environment, free from pathogens.
iii. It can be protected against the nature’s hazards.
iv. From the germplasm stock, large number of plants can be obtained whenever needed.
ADVERTISEMENTS:
v. Obstacles for their transport through national and international borders are minimal (since the germplasm is maintained under aspectic conditions).
There are mainly three approaches for the in vitro conservation of germplasm:
1. Cryopreservation (freeze-preservation)
2. Cold storage
3. Low-pressure and low-oxygen storage
Cryopreservation:
Cryopreservation (Greek, krayos-frost) literally means preservation in the frozen state. The principle involved in cryopreservation is to bring the plant cell and tissue cultures to a zero metabolism or non-dividing state by reducing the temperature in the presence of cryoprotectants.
ADVERTISEMENTS:
Cryopreservation broadly means the storage of germplasm at very low temperatures:
i. Over solid carbon dioxide (at -79°C)
ii. Low temperature deep freezers (at -80°C)
iii. In vapour phase nitrogen (at -150°C)
iv. In liquid nitrogen (at -196°C)
Among these, the most commonly used cryopreservation is by employing liquid nitrogen. At the temperature of liquid nitrogen (-196°C), the cells stay in a completely inactive state and thus can be conserved for long periods.
ADVERTISEMENTS:
In fact, cryopreservation has been successfully applied for germplasm conservation of a wide range of plant species e.g. rice, wheat, peanut, cassava, sugarcane, strawberry, coconut. Several plants can be regenerated from cells, meristems and embryos stored in cryopreservation.
Mechanism of Cryopreservation:
The technique of freeze preservation is based on the transfer of water present in the cells from a liquid to a solid state. Due to the presence of salts and organic molecules in the cells, the cell water requires much more lower temperature to freeze (even up to -68°C) compared to the freezing point of pure water (around 0°C). When stored at low temperature, the metabolic processes and biological deteriorations in the cells/tissues almost come to a standstill.
Precautions/Limitations for Successful Cryopreservation:
Good technical and theoretical knowledge of living plant cells and as well as cryopreservation technique are essential.
Other precautions (the limitations that should be overcome) for successful cryopreservation are listed below:
i. Formation ice crystals inside the cells should be prevented as they cause injury to the organelles and the cell.
ii. High intracellular concentration of solutes may also damage cells.
iii. Sometimes, certain solutes from the cell may leak out during freezing.
iv. Cryoprotectants also affect the viability of cells.
v. The physiological status of the plant material is also important.
Technique of Cryopreservation:
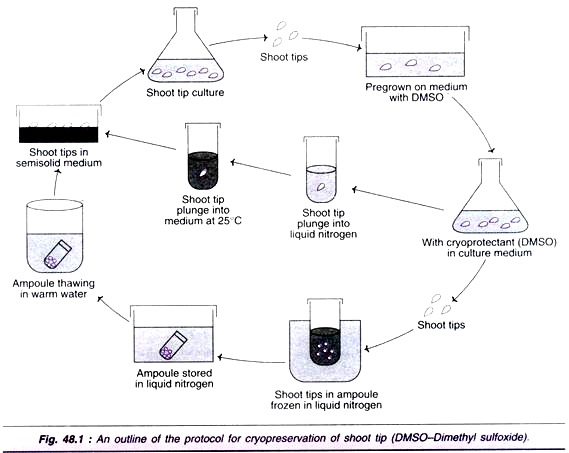
An outline of the protocol for cryopreservation of shoot tip is depicted in Fig. 48.1. The cryopreservation of plant cell culture followed by the regeneration of plants broadly involves the following stages
1. Development of sterile tissue cultures
2. Addition of cryoprotectants and pretreatment
3. Freezing
4. Storage
5. Thawing
6. Re-culture
7. Measurement of survival/viability
8. Plant regeneration.
The salient features of the above stages are briefly described.
Development of sterile tissue culture:
The selection of plant species and the tissues with particular reference to the morphological and physiological characters largely influence the ability of the explant to survive in cryopreservation. Any tissue from a plant can be used for cryopreservation e.g. meristems, embryos, endosperms, ovules, seeds, cultured plant cells, protoplasts, calluses. Among these, meristematic cells and suspension cell cultures, in the late lag phase or log phase are most suitable.
Addition of cryoprotectants and pretreatment:
Cryoprotectants are the compounds that can prevent the damage caused to cells by freezing or thawing. The freezing point and super-cooling point of water are reduced by the presence of cryoprotectants. As a result, the ice crystal formation is retarded during the process of cryopreservation.
There are several cryoprotectants which include dimethyl sulfoxide (DMSO), glycerol, ethylene, propylene, sucrose, mannose, glucose, proline and acetamide. Among these, DMSO, sucrose and glycerol are most widely used. Generally, a mixture of cryoprotectants instead of a single one is used for more effective cryopreservation without damage to cells/tissues.
Freezing:
The sensitivity of the cells to low temperature is variable and largely depends on the plant species.
Four different types of freezing methods are used:
1. Slow-freezing method:
The tissue or the requisite plant material is slowly frozen at a slow cooling rates of 0.5-5°C/min from 0°C to -100°C, and then transferred to liquid nitrogen. The advantage of slow-freezing method is that some amount of water flows from the cells to the outside. This promotes extracellular ice formation rather than intracellular freezing. As a result of this, the plant cells are partially dehydrated and survive better. The slow-freezing procedure is successfully used for the cryopreservation of suspension cultures.
2. Rapid freezing method:
This technique is quite simple and involves plunging of the vial containing plant material into liquid nitrogen. During rapid freezing, a decrease in temperature -300° to -1000°C/min occurs. The freezing process is carried out so quickly that small ice crystals are formed within the cells. Further, the growth of intracellular ice crystals is also minimal. Rapid freezing technique is used for the cryopreservation of shoot tips and somatic embryos.
3. Stepwise freezing method:
This is a combination of slow and rapid freezing procedures (with the advantages of both), and is carried out in a stepwise manner. The plant material is first cooled to an intermediate temperature and maintained there for about 30 minutes and then rapidly cooled by plunging it into liquid nitrogen. Stepwise freezing method has been successfully used for cryopreservation of suspension cultures, shoot apices and buds.
4. Dry freezing method:
Some workers have reported that the non-germinated dry seeds can survive freezing at very low temperature in contrast to water-imbibing seeds which are susceptible to cryogenic injuries. In a similar fashion, dehydrated cells are found to have a better survival rate after cryopreservation.
Storage:
Maintenance of the frozen cultures at the specific temperature is as important as freezing. In general, the frozen cells/tissues are kept for storage at temperatures in the range of -70 to -196°C. However, with temperatures above -130°C, ice crystal growth may occur inside the cells which reduces viability of cells. Storage is ideally done in liquid nitrogen refrigerator — at 1 50°C in the vapour phase, or at -196°C in the liquid phase.
The ultimate objective of storage is to stop all the cellular metabolic activities and maintain their viability. For long term storage, temperature at -196°C in liquid nitrogen is ideal. A regular and constant supply of liquid nitrogen to the liquid nitrogen refrigerator is essential. It is necessary to check the viability of the germplasm periodically in some samples. Proper documentation of the germplasm storage has to be done.
The documented information must be comprehensive with the following particulars:
i. Taxonomic classification of the material
ii. History of culture
iii. Morphogenic potential
iv. Genetic manipulations done
v. Somaclonal variations
vi. Culture medium
vii. Growth kinetics
Thawing:
Thawing is usually carried out by plunging the frozen samples in ampoules into a warm water (temperature 37-45°C) bath with vigorous swirling. By this approach, rapid thawing (at the rate of 500- 750°C min-1) occurs, and this protects the cells from the damaging effects ice crystal formation.
As the thawing occurs (ice completely melts) the ampoules are quickly transferred to a water bath at temperature 20-25°C. This transfer is necessary since the cells get damaged if left for long in warm (37-45°C) water bath. For the cryopreserved material (cells/tissues) where the water content has been reduced to an optimal level before freezing, the process of thowing becomes less critical.
Re-culture:
In general, thawed germplasm is washed several times to remove cryoprotectants. This material is then re-cultured in a fresh medium following standard procedures. Some workers prefer to directly culture the thawed material without washing. This is because certain vital substances, released from the cells during freezing, are believed to promote in vitro cultures.
Measurement of survival/viability:
The viability/survival of the frozen cells can be measured at any stage of cryopreservation or after thawing or re-culture.
The techniques employed to determine viability of cryopreserved cells are the same as used for cell cultures .Staining techniques using triphenyl tetrazolium chloride (TTC), Evan’s blue and fluorescein diacetate (FDA) are commonly used.
The best indicator to measure the viability of cryopreserved cells is their entry into cell division and regrowth in culture. This can be evaluated by the following expression.
Plant regeneration:
The ultimate purpose of cryopreservation of germplasm is to regenerate the desired plant. For appropriate plant growth and regeneration, the cryopreserved cells/tissues have to be carefully nursed, and grown. Addition of certain growth promoting substances, besides maintenance of appropriate environmental conditions is often necessary for successful plant regeneration.
A selected list of plants (in various forms) that have been successfully used for cryopreservation is given in Table 48.1.
Cold Storage:
Cold storage basically involves germplasm conservation at a low and non-freezing temperatures (1-9°C) The growth of the plant material is slowed down in cold storage in contrast to complete stoppage in cryopreservation. Hence, cold storage is regarded as a slow growth germplasm conservation method. The major advantage of this approach is that the plant material (cells/tissues) is not subjected to cryogenic injuries.
Long-term cold storage is simple, cost-effective and yields germplasm with good survival rate. Many in vitro developed shoots/plants of fruit tree species have been successfully stored by this approach e.g. grape plants, strawberry plants.
Virus- free strawberry plants could be preserved at 10°C for about 6 years, with the addition of a few drops of medium periodically (once in 2-3 months). Several grape plants have been stored for over 15 years by cold storage (at around 9°C) by transferring them yearly to a fresh medium.
Low-Pressure and Low-Oxygen Storage:
As alternatives to cryopreservation and cold storage, low-pressure storage (LPS) and low-oxygen storage (LOS) have been developed for germplasm conservation. A graphic representation of tissue culture storage under normal atmospheric pressure, low-pressure and low-oxygen is depicted in Fig. 48.2.
Low-Pressure Storage (LPS):
In low-pressure storage, the atmospheric pressure surrounding the plant material is reduced. This results in a partial decrease of the pressure exerted by the gases around the germplasm. The lowered partial pressure reduces the in vitro growth of plants (of organized or unorganized tissues). Low-pressure storage systems are useful for short-term and long-term storage of plant materials.
The short-term storage is particularly useful to increase the shelf life of many plant materials e.g. fruits, vegetables, cut flowers, plant cuttings. The germplasm grown in cultures can be stored for long term under low pressure. Besides germplasm preservation, LPS reduces the activity of pathogenic organisms and inhibits spore germination in the plant culture systems.
Low-Oxygen Storage (LOS):
In the low-oxygen storage, the oxygen concentration is reduced, but the atmospheric pressure (260 mm Hg) is maintained by the addition of inert gases (particularly nitrogen). The partial pressure of oxygen below 50 mm Hg reduces plant tissue growth (organized or unorganized tissue). This is due to the fact that with reduced availability of O2, the production of CO2 is low. As a consequence, the photosynthetic activity is reduced, thereby inhibiting the plant tissue growth and dimension.
Limitations of LOS:
The long-term conservation of plant materials by low-oxygen storage is likely to inhibit the plant growth after certain dimensions.
Applications of Germplasm Storage:
The germplasm storage has become a boon to plant breeders and biotechnologists.
Some of the applications are briefly described:
1. Maintenance of stock cultures: Plant materials (cell/tissue cultures) of several species can be cryopreserved and maintained for several years, and used as and when needed. This is in contrast to an in vitro cell line maintenance which has to be sub-cultured and transferred periodically to extend viability. Thus, germplasm storage is an ideal method to avoid sub-culturing, and maintain cells/ tissues in a viable state for many years.
2. Cryopreservation is an ideal method for long term conservation of cell cultures which produce secondary metabolites (e.g. medicines).
3. Disease (pathogen)-free plant materials can be frozen, and propagated whenever required.
4. Recalcitrant seeds can be maintained for long.
5. Conservation of somaclonal and gametoclonal variations in cultures.
6. Plant materials from endangered species can be conserved.
7. Conservation of pollen for enhancing longevity.
8. Rare germplasms developed through somatic hybridization and other genetic manipulations can be stored.
9. Cryopreservation is a good method for the selection of cold resistant mutant cell lines which could develop into frost resistant plants.
10. Establishment of germplasm banks for exchange of information at the international level.
Limitations of Germplasm Storage:
The major limitations of germplasm storage are the expensive equipment and the trained personnel. It may, however, be possible in the near future to develop low cost technology for cryopreservation of plant materials.