ADVERTISEMENTS:
Over the years, a number of sophisticated analytical and preparative techniques have been developed for separating, analyzing, and isolating the various macromolecular constituents of cells and tissues.
Various forms of electrophoresis, chromatography, and ultracentrifugation are now in routine use in most laboratories engaged in molecular biological studies and have greatly increased our understanding of the chemistry and properties of the cellular macromolecules.
Most of the methods that are used to separate and isolate different members of a class of macromolecules simultaneously provide information concerning their chemistry because parameters such as molecular size, shape, density, net and absolute charge, differential solubility, and so forth are used as the basis for the separation.
ADVERTISEMENTS:
Nearly all the techniques that are used for separating and isolating macromolecules require that these substances initially be in a soluble state.
Consequently, macromolecules present in extracellular fluids such as plasma, lymph, and hormonal and digestive secretions are most easily isolated and until recently were the subject of the most intensive studies.
Now, however, a variety of procedures are available to solubilize macromolecular constituents of membranes and other particulate components of cells. Therefore, in recent years, there has been a concerted effort to isolate and chemically characterize these macromolecular constituents as well.
A number of physical methods were described for disrupting cells. Often, more vigorous or more extensive applications of the same procedures to whole cells or to isolated organelles will also free some of the constituent macromolecules. For example, extended sonification or homogenization of mitochondrial suspensions solubilizes many of the mitochondrial enzymes and other constituents, and nucleic acids may be released from isolated nuclei under similar conditions.
ADVERTISEMENTS:
Chemical procedures may also be used to extract or solubilize the desired class of macromolecules. Lipids are often extracted from whole cells or isolated organelles using organic solvents such as chloroform-methanol mixtures or acetone.
Proteins may be extracted from membranous elements using dissociating agents (such as urea and mercapto- ethanol), chelating agents (such as ethylenediamine tetraacetic acid, EDTA) or organic detergents (such as sodium deoxycholate, sodium dodecyl sulfate, and Triton X-100). Whatever the method used, once a soluble mixture of macromolecules is obtained, these may then be separated from one another and isolated using one or a combination of methods.
A number of methods that are either in routine use by cell biologists for isolating and characterizing cellular macromolecules (proteins, carbohydrates, lipids, and nucleic acids) or of significant historical interest will be considered. Most of these methods (see Table 13-1) rely on differences in molecular size, shape, electrostatic charge, solubility, or biological activity to effect the separation.
Salting In and Salting Out:
Among the oldest methods for fractionating and isolating mixtures of macromolecules (especially proteins) are chemical procedures that differentially alter a molecule’s solubility. Among other factors, a protein is maintained in the dissolved (i.e., solubilized) state by the interaction of its charged groups with water (which is partially polar) and with salt ions in the solution.
The salt concentration (or ionic strength) of the protein solution significantly and differentially influences the solubility of the proteins present. For example, many proteins are insoluble in pure (i.e., salt-free) water, whereas addition of small quantities of salt renders these molecules soluble.
This effect is believed to be due to an interaction between the salt ions and certain charged groups of the protein that would otherwise react with each other, resulting in insolubility. Even proteins that are soluble in distilled water may be dissolved in much greater quantities by the simultaneous addition of small amounts of salts. This phenomenon is known as salting in.
If the salt concentration of a protein solution is successively increased, a point is eventually reached at which some of the proteins begin to precipitate. Further addition of salt results in greater precipitation. Precipitation occurs because spheres of hydration formed around the salt ions effectively “remove” the water molecules necessary to hydrate certain surface charges of the protein.
Protein- protein interactions begin to dominate over protein- solvent interactions with the resulting precipitation of the proteins. In effect, the proteins are being “squeezed out of solution.” This phenomenon is called salting out. The effect of salt concentration on the solubility of hemoglobin is shown in Figure 13-1.
Isoelectric Precipitation:
The distribution of polar groups in proteins and nucleic acids is influenced by pH. For a given protein, a pH may be identified at which there are equal amounts of positive and negative charge, and this is known as the isoelectric point (or isoelectric pH).
Most proteins are least soluble and many proteins are insoluble at the isoelectric point, so that their removal from solution is most easily achieved by first adjusting the pH of the solution to the isoelectric point.
Dialysis and Ultrafiltration:
Semipermeable membranes, such as those prepared from cellophane or collodion (cellulose nitrate) may be used to separate solutes on the basis of molecular weight differences. The technique is called dialysis and was first described in 1861 by T. Graham. In dialysis, the solute mixture is placed in a bag formed from tubular sheets of the semipermeable membrane and the bag is immersed in an aqueous medium (e.g., distilled water).
Molecules larger than the pores of the membrane are confined to the tubing, whereas smaller molecules diffuse into the surrounding liquid (Fig. 13-2). Semipermeable membranes can be treated chemically or physically to alter the sizes of the pores so that solutes of varying molecular weight are rendered permeable.
Generally, dialysis is used with unmodified membranes to quickly separate low- molecular-weight solutes (i.e., molecular weight of less than 5000) such as salts, sugars, and amino acids from proteins and polysaccharides present in the solution. However, high-molecular-weight solutes do differentially penetrate membranes having large pore sizes (Table 13-2).
In ultrafiltration, force is used to drive the smaller molecules along with solvent through the semipermeable membrane. As a result, not only are permeable and impermeable molecules separated, but the impermeable species is also simultaneously concentrated (Fig. 13-3).
Ultracentrifugation:
Centrifugation can be employed not only for the separation of cells, subcellular organelles, and other particulate constituents of cells but also for molecular separations. Since its initial development in the 1920s by T. Svedberg, the analytical ultracentrifuge has been used repeatedly to evaluate the heterogeneity or purity of molecular constituents extracted from cells and to estimate molecular sizes on the basis of sedimentation rate.
ADVERTISEMENTS:
Preparative separations (as opposed to analytical studies) became possible with the development of ultracentrifuges capable of spinning rotors that could hold large amounts of sample and that could attain speeds producing an RCF in excess of 500,000 g. Using forces of this magnitude, true separations and isolations of molecular constituents of cells in rate or isopycnic gradients have become routine.
However, it should be noted that certain problems are encountered during the fractionation of macromolecules in density gradients that do not arise during particle or organelle fractionations.
Most important among these problems is diffusion. Macromolecules (or, for that matter, any solute) in solution exhibit a net migration away from any region in which they are concentrated and toward regions containing a lower concentration of the solute. This movement, called diffusion, results in the uniform distribution of molecules throughout the space that they occupy.
ADVERTISEMENTS:
The rate at which diffusion occurs is given by Ficke’s law:
dS/dt = -D(dC/dx) (13-1)
In which S is the amount of substance undergoing diffusion, C is the concentration of the diffusing solute, t is time, x is the distance through which diffusion occurs, and D is a constant (called the diffusion constant) that characterizes the behavior of the solute molecule in a given solvent.
It should be noted that the rate of diffusion is proportional to the concentration of diffusing solute, so that the tendency for diffusion increases as the concentration of the diffusing species increases. It follows that the banding of a macromolecule in a density gradient is an attempt to concentrate that molecule and is increasingly opposed by the diffusion of that molecule.
For large cellular particles or organelles, the effects of diffusion are negligible because the diffusion constants of large particles are so small. However, the diffusion constants of proteins, nucleic acids, and other macromolecules are considerably greater; consequently, during centrifugation, equilibrium between sedimentation and diffusion is eventually reached, with the result that no further concentration of a given molecular species by isopycnic banding is possible.
In effect, diffusion places a limit on the resolution attainable when separating macromolecular mixtures in density gradients. Diffusion also influences the effectiveness of other methods of macromolecular fractionation, especially dialysis.
Electrophoresis:
The term electrophoresis originally described the migration of charged particles through a liquid or semisolid medium under the influence of an electrical potential. More recently, the word has come to refer to any technique by which molecules are separated from one another in electrical potential gradients on the basis of differences in their net charges, sizes, and shapes, regardless of the conducting medium.
ADVERTISEMENTS:
The method is employed most often for the separation of different proteins, as the side chains of many of the constituent amino acids exist in a dissociated form and contribute some number of positive and negative charges to the macromolecule. However, other molecules such as polynucleotides are also effectively separated by electrophoresis.
The fundamental principles of electrophoresis are quite simple. If two electrodes are inserted into a solution containing an electrolyte and a suspension of macromolecules of varying net charge, the macromolecules will be accelerated toward the electrode of opposite sign with a force proportional to the magnitude of the charge on the macromolecule (which is pH dependent) and the strength of the applied electrical potential.
Because each of the migrating particles will encounter frictional resistance to its movement, each will soon attain some maximum velocity of migration. Therefore, if a mixture of these macromolecules is exposed to constant field strength, the maximum velocities attained will be different for particles of differing net charge.
In addition to net charge and field strength, several other factors influence the rate of electrophoretic migration of proteins and polynucleotides, including molecular size and shape and the nature of the medium through which migration occurs. These factors will be considered later.
The rate at which a protein migrates toward one or the other electrode during electrophoresis is called its electrophoretic mobility (usually expressed in cm2 sec-1 V-1) and is dependent on the relative numbers of positively charged and negatively charged amino acid side chains. Whether or not a particular amino acid side chain carries a charge is, in turn, determined in part by the pH of the protein solution.
ADVERTISEMENTS:
As the pH is lowered (i.e., the concentration of H+ is increased), negatively charged groups such as the secondary COO” of aspartic and glutamic acid and the O– of tyrosine become protonated, thereby neutralizing these negative charges. At the same time, some secondary amino groups such as those of lysine and arginine may accept additional protons, thereby increasing the number of positive charges associated with the protein.
In contrast, as the pH is raised (i.e., the concentration of OH– is increased), protons are dissociated from these side chains and make the protein more negative. These relationships are shown in Figure 13-4. Therefore, the numbers and types of charges associated with the amino acid side chains of a protein are determined by pH.
At low pH, proteins tend to carry more positive than negative side chains and, therefore, possess a net positive charge and migrate toward the cathode (the negative electrode) during electrophoresis. At high pH, negatively charged side chains predominate and the protein migrates toward the anode.
It follows from the above discussion that for every protein there will be a pH at which the total positive charge equals the total negative charge. If electrophoresis is carried out at this pH, no migration occurs, because the protein has no net charge.
Above this pH, the net charge on the protein becomes increasingly negative, and its electrophoretic mobility toward the anode increases. Below this pH, electrophoretic mobility toward the cathode increases. The relationships between pH and electrophoretic mobility for the proteins egg albumin and plasma β – lactoglobulin are shown in Figure 13-5.
The pH at which a protein possesses no electrophoretic mobility is called the isoelectric point and is a characteristic of each protein (Table 13-3). Because pH markedly influences electrophoretic mobility, it is important to maintain a constant electrolyte pH during electrophoresis. This is accomplished by including buffers in the electrolyte solutions.
Moving-Boundary Electrophoresis:
Electrophoresis of proteins was introduced in its original form by A. Tiselius in 1937 and called “moving- boundary” electrophoresis. For his achievements and his contributions to biochemistry through the development of electrophoretic and chromatographic procedures, Tiselius received the 1948 Nobel Prize in Chemistry. To separate the different proteins in a mixture, the sample (dissolved in a buffer solution that served as the electrolyte and maintained the desired pH) was placed in a glass tube connected to electrodes.
When an electrical potential was applied across the tube, the protein molecules migrated toward one or the other electrode. Because different proteins migrate at different rates, a number of interfaces or boundaries formed between the leading (and trailing) edge of each protein type and the remaining mixture.
A Schlieren optical system recorded the number of boundaries formed and their rates of migration. The moving- boundary technique was used for the analysis but not for the fractionation of complex mixtures of proteins, as the proteins in the mixture were not really separated from each other.
Zone Electrophoresis:
Zone electrophoresis offers a number of important advantages over moving-boundary electrophoresis. In zone electrophoresis, the separation of the different proteins in a sample is in fact realized. For this reason, the technique can be both analytical and preparative.
Generally, zone electrophoresis also yields greater resolution of the protein components of a mixture. In zone electrophoresis, the proteins are separated in a semisolid or porous supporting medium such as filter paper or various types of gels (polyacryl- amide, cellulose acetate, hydrolyzed starch, etc.); these usually take the form of narrow sheets, slabs, or columns.
The principles of zone electrophoresis may be illustrated by considering the filter paper technique as an example. The strip of filter paper is saturated with the buffer/electrolyte solution to be used and is tautly suspended between two (inner) baths (Fig. 13-6).
The inner baths communicate with two additional (outer) baths containing the electrodes; the connections may be achieved using baffles or wicks saturated with the buffer/electrolyte. The electrodes are not inserted directly into the inner baths because electrolysis would dramatically alter the pH of the bath and also the filter paper strip. By confining the electrolysis to the outer baths, the pH of the inner baths and the filter paper remains constant. The mixture of proteins to be separated is applied as a narrow zone perpendicular to the long axis of the filter paper.
When an electrical potential is applied, the proteins in the sample zone migrate through the filter paper toward the appropriate electrode. In so doing, they form a number of discrete zones distributed along the length of the paper (Fig. 13-6). Following separation, the individual zones or bands can be visualized using special stains. Alternatively, the paper strip may be cut into a number of sections and the proteins in each eluted and collected for further study.
Because the medium through which the proteins migrate is porous, the size of the protein influences its electrophoretic mobility—smaller proteins migrating more rapidly than larger proteins of equal net charge. Consequently the sieving effect of the supporting medium results in a separation based on both molecular size and charge.
Polyacrylamide gels are especially effective for zone electrophoretic separations, because the sieving effect may be varied by changing the concentration of acrylamide used to prepare the gel (i.e., the greater the acrylamide concentration, the smaller the pore size in the gel).
An interesting modification of zone electrophoresis is diagonal electrophoresis or two-dimensional electrophoresis. In this approach, the sample is separated first on a rectangular sheet of filter paper or polyacrylamide gel at high pH; then, after the supporting medium is rotated 90° and equilibrated at low pH, the separated zones are electrophoresed again. The result is a better resolved distribution of the components in the original sample, as it is highly unlikely that two components would have the same electrophoretic mobility at grossly different pHs.
Disk or Discontinuous Electrophoresis:
Discontinuous electrophoresis is a specialized form of zone electrophoresis developed in the early 1960s by L. Ornstein and B. Davis. In view of its widespread use and importance as a research tool, it will be considered separately. The procedure provides the highest degree of resolution so far attainable by electrophoretic methods, and this is a consequence of the extreme thinness of the sample zone.
Disk electrophoresis is carried out in either columns or sheets of polyacrylamide gel, the pore sizes of which may be accurately controlled by regulating the concentration of acrylamide in the gel. Consequently, the gel acts like a molecular sieve and separation is based on both the charge and the size of the macromolecule.
To separate two proteins by conventional methods of zone electrophoresis, it is necessary to permit migration to continue until one of the proteins has traveled at least one starting zone thickness further than the other. For example, suppose that the sample zone was 1mm thick and contained two different proteins. If one of the proteins has no electrophoretic mobility at all under the pH conditions employed, then the other would have to migrate at least 1 mm for separation of the two proteins to occur.
If both proteins migrate in the same direction but at different velocities, then an even greater distance must be traversed by the protein of higher electrophoretic mobility. Because the sharpness of the zone occupied by each protein decreases with time owing to diffusion in the gel, it is most desirable to begin with the narrowest starting zone possible and to minimize the duration of electrophoresis.
In disk electrophoresis, the sample zone undergoes a preliminary concentration during which its thickness is reduced from one or more centimeters to just a few hundredths of a centimeter; as a result, high resolution is achieved during very brief runs. The preliminary concentration also makes it possible to analyze samples too small or too dilute to be studied using other methods.
The ability to concentrate and .narrow the starting zone during disk electrophoresis is based on a phenomenon first noted and described by F. Kohlrausch in 1897. He showed that if two solutions of ions having significantly different electrophoretic mobilities are layered over one another (e.g., slow ion above, fast ion below) and subjected to an electrical field, the boundary-between the two ionic species would be sharply maintained as the ions migrate.
That is, under these conditions the ions of lower electrophoretic mobility migrate at the same rates as the faster ions. This may be explained as follows. The velocity at which an ion migrates in an electrical field is determined by the product of its electrophoretic mobility and the applied voltage gradient (V/cm).
Therefore, ions of low mobility can migrate as rapidly as ions of high mobility if their mobility-voltage products are equal. In the example cited above, at the instant voltage is applied, the trailing edge of the fast ions at the boundary moves away from the leading edge of the slow ions, resulting in the temporary formation of a zone of reduced conductivity and increased field strength. The increased field strength in this zone accelerates the slow ions to keep up with the fast ions, thereby creating a steady state in which the mobility-voltage products of the two ions remain constant.
Taking this a step further, consider the situation in which a region below the fast ion-slow ion boundary contains a number of ions of intermediate electrophoretic mobility (Fig. 13-7a). When an electrical potential is applied, the increased voltage gradient behind the downward-moving boundary accelerates both the ions of low mobility and those of intermediate mobility.
The mobility-voltage product of the latter, however, will be somewhat greater, so that these ions will be swept up by the boundary and form a zone of continuously decreasing thickness between the fast and slow ions (Figs. 13-7b, 13-7c, and 13-7d).
If instead of a single ionic species of intermediate mobility, a mixture of ions is placed in the region below the slow ion- fast ion boundary these would be concentrated into narrow zones stacked one above the other in order of decreasing mobility. In disk electrophoresis, conditions are chosen such that the proteins in the sample have mobilities intermediate to two especially selected fast and slow ions. Disk electrophoresis gels are cylindrical blocks or flat sheets of polyacrylamide suspended between two reservoirs of buffer/electrolyte (Fig. 13-8). Usually, the polyacrylamide gel is divided into three regions called the sample gel, stacking gel, and separating gel.
The sample gel contains the mixture of proteins to be separated and is prepared using low concentrations of acrylamide so that pore sizes are large and do not influence the rates of migration of different size proteins. The stacking gel is similar to the sample gel but lacks the proteins. The sample gel, stacking gel, and reservoirs have the same pH (usually 8.3).
The separating gel differs from the other two regions in that it has a higher pH (usually 9.5) and is prepared with greater concentrations of acrylamide; this results in smaller pore sizes and provides the sieving effect. All three regions of the polyacrylamide gel contain the fast (leading) ion, whereas the buffer/electrolyte contains the slow (trailing) ion.
In most instances, Cl– serves as the fast ion and glycine (NH2CH2COO–), as the slow ion; other amino acids or weak acids may also serve as the slow ion. At pH 8.3, nearly all proteins have an electrophoretic mobility between those of Cl– and glycine.
When an electrical potential is applied across the gel, the voltage gradient causes the chloride ions to migrate down from the top of the gel. The increased field strength created immediately behind the trailing edge of the chloride ions accelerates the slower glycine ions to keep pace.
As the CI–-glycine boundary moves downward, it overtakes the more slowly migrating proteins, and they too are accelerated by the increased field strength behind the boundary. As a result, by the time the trailing edge of the chloride ions reaches the end of the stacking gel, all the proteins in the original sample have been concentrated into a series of contiguous thin zones.
Once the separating gel is reached, the change in pH dramatically alters the electrophoretic mobility of the trailing ion. In the case of glycine, the degree of ionization is very low at pH 8.3 but is several times greater at pH 9.5. As a result, the glycine ions now overtake each of the protein zones and catch up with the trailing edge of the CI– (the mobility of Cl– is unaffected by the pH change), and the new interface then migrates rapidly through the remainder of the separating gel.
The proteins, on the other hand, are physically retarded by the smaller pores in the separating gel, and because each protein zone is now in a uniform electrical field, these separate from one another strictly on the basis of net charge and size difference (just as in conventional zone electrophoresis). It is apparent from the foregoing discussion that disk electrophoresis differs markedly from conventional zone electrophoresis and is a clever and unusual technique that provides extraordinary resolution (Fig. 13-9).
The method has been widely accepted since its introduction and has been applied in diverse cell studies, especially in the analysis of the protein components of isolated cell organelles. An example of this is given in Figure 13-10, which shows more than 30 different protein components separated from the plasma membranes of liver cells.
SDS-PAGE SDS (sodium dodecyl sulfate) is an ionic detergent that has been used for many years to solubilize membrane proteins so that they can be separated either electrophoretically or by other methods. Because membrane proteins are not usually soluble in aqueous solutions, the SDS is also incorporated into the separating medium; this prevents the proteins from forming an intractable precipitate once they enter the gel. Because the separating medium used with the SDS is polyacrylamide gel, the technique is referred to as SDS-PAGE (i.e., SDS-polyacrylamide gel electrophoresis).
SDS dissociates oligomeric proteins into their subunits and forms ionic bonds with the polypeptides. Therefore, the SDS alters the protein’s native electrostatic charge and adds to its mass. As a result, when electrophoresis is carried out in small-pore polyacrylamide gels containing SDS, the proteins migrate through the gel at rates determined principally by molecular size (the smaller polypeptides traveling farther through the gel than the larger polypeptides).
If in two neighboring tracks or lanes of the same gel, proteins of known molecular weights are electrophoresed alongside a sample containing proteins of unknown molecular weights, the latter’s molecular weights can be estimated by comparing their final positions with those of the “markers.” Hence, SDS-PAGE is a powerful tool for both separating and sizing macromolecules.
The Maxam-Gilbert Technique:
In recent years, the SDS-PAGE method has been extended to the separation of polynucleotide fragments obtained by enzymatic cleavage of DNA and RNA. Not only are the polynucleotide fragments separated on the basis of size differences, but the order of the nitrogenous bases in the original DNA or RNA molecule is revealed. The technique is known by the names of its developers, A. Maxam and W. Gilbert. To illustrate this ingenious and most valuable procedure, suppose that the base sequence of the following polynucleotide is to be determined:
5′-CTACGTAG-3′:
The polynucleotides are subjected to enzymatic cleavage using encdonucleases that introduce breaks on the 5′ side of specific bases, but the amounts of the en- donucleases and the lengths of the exposure times permit only one cleavage per polynucleotide.
As a result, the following fragments are formed:
1. Cleavage at A yields 5′-CT-3′ and 5′-CTACGT-3′ fragments.
2. Cleavage at T yields 5′-C-3′ and 5′-CTACG-3′ fragments.
3. Cleavage at G yields 5′-CTAC-3′ and 5′- CTACGTA-3′ fragments
4. Cleavage at C yields 5′-CTA-3′ fragments.
Other fragments are also formed, but they are not radioactive because they do not contain the original 5′ ends.
When the four families of fragments are electrophoresed in neighboring lanes of an SDS-poly-acrylamide gel, the resulting pattern of radioactive fragments revealed by autoradiography takes the form shown in Figure 13-11. Fragment 5′- CTACGTA-3′ produced by cleavage at G migrates the most slowly because it is the largest fragment.
Therefore, lane G has the slowest band; migrating more rapidly is a band in the A lane, then the T lane, and so on. Reading in order beginning at the top of the pattern, we obtain GATGCAT, which is the base sequence of the polynucleotide beginning at its 3′ end. Using the Maxam-Gilbert technique, the base sequences in fragments containing several hundred nucleotides are readily determined (Figure 13-11b).
Immunoelectrophoresis:
Immunoelectrophoresis, a technique developed in the 1950s by C. A. Williams and R Grabar, combines the principles of electrophoresis and immunochemistry to separate and identify antigens and antibodies. In this technique, a portion of the sample of antigens to be analyzed is first injected into an experimental animal (usually a horse or cow).
After a period of time during which the animal produces antibodies against the injected antigens, the immunoglobulin-rich blood serum is collected and prepared as the antiserum. The remaining portion of the sample to be analyzed is then subjected to conventional zone electrophoresis in a rectangular block of agar or other supporting medium. This distributes the various proteins in the original sample into a linear series of zones (Fig. 13-12a). A trough cut in the agar block parallel to the direction of electrophoretic migration is then filled with the antiserum.
The protein zones in the agar block diffuse radially and eventually encounter the front of inwardly diffusing antibody, where the resulting antibody-antigen reaction produces a fine, arc- shaped line of precipitation (Fig. 13-12d). Each line of precipitation corresponds to a pair of precipitants (a protein in the sample plus an antibody from the antiserum); crossed arcs indicate that all the reactants are immunochemically different proteins.
Isoelectric Focusing:
Isoelectric focusing is a form of electrophoresis used to determine the isoelectric pHs of proteins and to separate proteins from one another on the basis of differences in their isoelectric points. The method was developed in the 1960s by H. Svensson and O. Vester- berg and is extremely sensitive; proteins having isoelectric pHs that differ by as little as 0.0025 units may be resolved.
In one form of isoelectric focusing, a glass column is filled with a solution of synthetic charged ampholytes (aliphatic amino-carboxylic acids of various molecular weights) having a broad and continuous range of isoionic points. The ends of the column contain the electrodes and electrode solutions: a strong organic base for the cathode (usually ethanol- amine) and a strong acid for the anode (usually phosphoric acid).
The distribution of constituents in the column is stabilized by the presence of a sucrose density gradient that also prevents convection during electrophoresis. The mixture of proteins to be separated may initially be confined to a specific region of the column or uniformly distributed through the density gradient (Fig. 13-13).
When a potential is applied to the electrodes, the ampholytes migrate in the column to positions where they are equally attracted by both electrodes. Thus, they arrange themselves in order of their isoelectric points, the most acidic ampholytes being located near the anode and the most basic near the cathode.
An examination of the pH in different regions of the column at the time would reveal that a pH gradient has been established in which pH increases in progressing from the anode to the cathode. Depending on the pH of the surrounding solution and the nature of the amino acid side chains present in the molecule, each protein assumes a characteristic net charge.
Proteins close to the anode will bear a net positive charge and be repelled by that electrode, whereas the same proteins near the cathode carry net negative charges and are repelled by that electrode. As a result, most proteins will migrate away from the electrodes and toward the center of the column. As this occurs, the proteins pass through the pH gradient and the charges on their amino acid side chains are altered. Proteins migrating away from the anode become less and less positive as they pass through regions of increasing pH, whereas proteins migrating away from the cathode become less negative (see Fig. 13-13).
Eventually, the net charge of each protein becomes zero as that region of the pH gradient corresponding to the isoelectric pH is reached, and at that time the electrophoretic migration of the protein ceases. Consequently, the proteins become distributed through the gradient as a series of narrow zones in order of their isoelectric points (see Fig. 13-13).
Once the migration of all proteins terminates, the contents of the column may be drained and the separated proteins collected as a series of fractions and further studied. The isoelectric point of each protein is determined from the pH profile of the collected gradient. For analytical applications, the liquid density gradient is replaced by a solid medium such as polyacrylamide gel. Several different samples, including standards of reference, can be focused in neighboring lanes of the gel.
A description of all electrophoretic methods currently in use is beyond the scope of this discussion. However, it should be recognized that other forms of electrophoresis such as density gradient electrophoresis and continuous-flow electrophoresis are also regularly employed.
In density gradient electrophoresis, the separation of proteins is carried out in a glass column filled with buffer and stabilized by a density gradient. Continuous-flow electrophoresis provides for the continuous application of small volumes of sample onto the supporting medium, coupled with the continuous removal of the proteins as separation is achieved. Both zone electrophoresis and isoelectric focusing have continuous-flow formats. With this technique it is possible to process very large volumes of starting material.
Although all methods of electrophoresis have proven extremely valuable for the separation and identification of proteins, certain limitations of the technique should be noted. The resolution of a protein mixture into a number of discrete zones does not guarantee that all the different proteins present in the original sample have been separated, for two or more different proteins having similar sizes may “also have the same net charge under a given set of conditions and display the same electrophoretic mobilities (see Fig. 13-5).
These proteins would not be resolved by electrophoresis. By the same token, two different proteins may have the same isoelectric pH and would not be separated by isoelectric focusing. Therefore, to evaluate the effectiveness of a separation and to determine the purity of the separated fractions, it is necessary to carry out electrophoresis under a variety of pH conditions or with a variety of supporting media or even to apply a combination of altogether different methods in the analysis.
Countercurrent Distribution:
However, the technique lends itself more directly to the separation of various molecular species in solution, and because the physicochemical principles in effect during countercurrent distribution are the foundation of many other separation procedures (i.e., paper chromatography, thin-layer chromatography, ion-exchange chromatography, affinity chromatography, gas chromatography, etc.), this method will be treated in some detail.
It has been known for a century or more that many solutes differentially distribute or partition themselves between the separated phases of two immiscible liquids. Solutes having gross differences in their physical properties may therefore be separated by one or a few simple extraction procedures (e.g., using separatory funnels).
Reasoning that the separation of a complex mixture of solutes on the basis of their partition differences could be made considerably more effective using a cascade of several dozen, several hundred, or even several thousand discrete extraction steps, L. C. Craig in the late 1940s and early 1950s developed a number of special instruments to achieve this. These instruments and their more modern counterparts are generally known as Craig apparatuses and the technique as countercurrent distribution.
The fundamental type of Craig apparatus consists of a series or train of chambers or “cells” divided into upper and lower compartments that house the respective immiscible liquid phases (e.g., butanol/water, phenol/water, propanol/water, etc.). The upper phase is usually the mobile phase and the lower phase is the stationary phase.
Equilibration of a dissolved solute between the phases within a chamber is achieved by agitation, so that one phase becomes finely dispersed in the other (thereby greatly increasing the interfacial surface area between the two phases). Once equilibration is achieved, the phases are allowed to separate.
The mobile phase is then transferred to the next chamber of the train and mixed with a fresh volume of stationary phase. Simultaneously, a fresh volume of mobile phase is added to the first chamber. Agitation, equilibration, separation, and transfer are then serially repeated for the entire train of chambers. A solute dissolved in a given pair of immiscible liquids will partition itself in a characteristic way, with a specific solute concentration ending up in each phase. The partition coefficient,
For example, if a solute has a partition coefficient of 1.0 in a butanol/water mixture, it will be equally divided between the butanol and the water phases once equilibration and separation have been achieved. Note that the solute is soluble in both liquids but partitions itself between the two in a characteristic way. Figure 13-14 depicts the countercurrent distribution of 128 molecules of a hypothetical solute having P = 1.0 when carried through a train consisting of seven sets of chambers. In this example, the peak of the solute distribution in the train occurs in chamber number 3, which contains 40 molecules (or 31%) of the original solute.
For a train consisting of (C +1) chambers (numbered C0, C1, C2, C3, etc.), each containing phases of equal volume, the fraction F of solute in compartment Cn at the conclusion of the countercurrent distribution is given by
where n is the number of transfers needed to reach chamber Cn (and is equal to the chamber number). For the example given in Figure 13-14, the fraction of solute in chamber C3 would be 6!(1.0)3/3!(6- 3)1(2.0)6, which equals 31%, as already noted. Figure 13-15 tabulates the final distributions of a mixture of five solutes having different P values through a train of seven chambers and illustrates how the peak concentrations of each solute would be found in different chambers in the train.
Although it may be noted that in this example considerable overlap exists among the chambers, it should be borne in mind that the number of chambers that make up the train has deliberately been kept low to simplify the example.
Usually, a train consists of at least several dozen chambers. Accordingly, Figure 13-16 shows the resolution attainable for solutes having P values of 0.5 and 2.0 when the train consists of 30 chambers. It can be shown that the best resolution of two solutes, A and B, is achieved when PA x PB = 1.0.
The number of chambers occupied by a particular solute as a function of the total number of chambers present in the train rapidly diminishes as n increases. Hence, it is clear why trains in which hundreds or even thousands of transfers are possible may be used to provide maximum resolution of the solutes present in the original mixture.
In a modified version of the original Craig approach (called countercurrent chromatography) a horizontal coiled length of tubing is filled with the stationary phase and the sample to be fractionated is introduced at one end. A mobile phase is then continuously pumped through the coil, progressively sweeping the sample toward the other end.
In each turn of the coil, partition of the sample between the stationary and mobile phases brings about a progressive fractionation of the original mixture. In one of its most elegant forms, the coil is oriented vertically and rotated around its own axis and/or a central axis so that centrifugal and hydrodynamic forces enhance the partition and the separation of the mobile and stationary phases.
The principles and mathematical relationships given above for a series of discrete partition transfers during countercurrent distribution apply equally well to seemingly quite distinct physical separation methods. In chromatography, the partition steps occur on numerous particles of tightly hydrated media such as starch, agarose, cellulose, silica, and paper fibers. This tightly bound solvent serves as the stationary phase and a mobile phase containing solvent and the solute mixture to be fractionated flows by.
This continuous process may be thought of as an infinite sequence of discrete, microscopic partition steps. Each microscopic step would be analogous to the stages shown in Figure 13-14, the upper mobile phase representing a continuously flowing solvent and the lower stationary phase the stationary, hydrated supporting medium. Although equilibrium is not truly achieved at each “step,” the number of steps is so great that the solute mixture is effectively fractionated.
Countercurrent distribution is particularly effective when dealing with molecules having molecular weights below 5000 (peptides, oligonucleotides, phospholipids, etc.), but the technique is also used successfully with larger molecules. The first protein isolated and purified using countercurrent distribution was the hormone insulin, which has a relatively low molecular weight (i.e., 6500).
However, ribonuclease (MW 13,600), lysozyme (MW 14,000), albumin (MW 68,000), and the alpha and beta chains (MW 16,000) of human hemoglobin have also been isolated using this approach. Little success is obtained with polysaccharides because of the problem of finding an organic phase that provides useful partition coefficient differences.
Ribonucleic acids are readily separated using countercurrent distribution; indeed, R. W. Holley’s pioneering analysis of the primary structure of transfer RNA was carried out using alanyl-tRNA isolated and purified by countercurrent distribution. Lipids, especially steroids, are well suited for separation using this approach because of their greater solubility in organic solvents and the wide choice of solvent combinations that can be used.
Paper Chromatography:
Paper chromatography is a technique in which a mixture of solutes is separated into discrete zones on a sheet of filter paper on the basis of (1) differences in solute partition between a stationary aqueous phase tightly bound to the cellulose fibers of the paper and a mobile organic liquid phase passing through the sheet by capillary action (i.e., liquid-liquid partition) and (2) differences in solute adsorption to the cellulose fibers and dissolution in the mobile liquid (i.e., solid-liquid partition).
Although the separation is based on a combination of both phenomena, liquid-liquid partition differences are the more significant. The principles of the technique were set down in the 1940s by R. Cons- den, A. H. Gordon, A. J. Martin, and R. L. Synge and are not unlike those that are in effect during counter- current distribution.
In practice, a rectangular sheet of filter paper is saturated with the aqueous phase and allowed to air dry, and the sample is applied near the end of the sheet as a narrow zone. The sheet is then suspended in a closed chamber in which the air has been saturated with the vapors of the mobile organic phase and the edge of the paper is immersed in a bath containing the mobile phase (Fig. 13-17a).
Capillary action causes the mobile phase to slowly percolate through the paper from one end (the end containing the mixture to be separated) to the other. Movement of the liquid may be downward (descending chromatography) or upward (ascending chromatography). The solute mixture differentially partitions itself between the flowing solvent and the stationary phase repeatedly as the solvent front advances toward the edge of the paper.
Usually, the solvent front is allowed to migrate through the paper until it has almost reached the other end, at which time the sheet is removed and dried and the solute zones located by the appropriate chemical or physical means. However, in certain instances where the solutes trail far behind the solvent front, it is desirable to allow the solvent to run off the edge of the paper sheet (descending chromatography only) so that maximum resolution of the solutes is achieved.
The rate of movement of a solute during paper chromatography is usually expressed as the dimensionless term Rf, which is the ratio of the distance traveled by the solute to the distance traveled by the solvent front. Naturally, the Rf can be calculated only in those instances when the solvent is not allowed to leave the end of the paper sheet.
Greater resolution of the solutes may be obtained using two-dimensional paper chromatography (Fig. 13-17b); after chromatography using a particular solvent system in one direction along the paper sheet, the sheet is dried and rotated 90°, and another solvent system is used to chromatograph the solutes a second time.
In this manner, solutes not fully separated by partition in the first solvent may be completely separated using the second solvent. Paper chromatography can also be combined with zone electrophoresis to provide two-dimensional analysis of a solute mixture, a technique known as “fingerprinting”.
Thin-Layer Chromatography:
Thin-layer chromatography (abbreviated TLC) is an especially valuable method for rapidly separating unsaturated and saturated fatty acids, triglycerides, phospholipids, steroids, peptides, nucleotides, and numerous other biological substances. In effect, TLC is a modification of paper chromatography in which the sheets of filter paper are replaced by glass or plastic plates covered with a thin, uniform layer of adsorbent.
The essential features of the technique may be described as follows:
An aqueous slurry of the selected adsorbent is uniformly spread over a glass plate to produce a thin layer and is then dried. Following this, the sample (usually prepared in a volatile solvent) is applied near one end of the long axis of the plate as a spot or thin line. When the sample has dried, the plate is supported vertically so that the end near the sample zone is immersed in a tray containing a shallow layer of the eluting solvent (usually an organic solvent of low polarity).
Capillary action causes the solvent to ascend slowly through the layer of adsorbent and, as in paper chromatography the solutes become distributed along the plate on the basis of differential partition between the stationary and mobile phases (Fig. 13-18). Although ascending TLC is the most common, descending and horizontal separations may also be carried out. TLC separations are very rapid, rarely exceeding 20 to 30 minutes.
After the separation has been achieved the glass plate is removed from the tray of solvent and allowed to dry. Zones containing colored substances can be detected directly, and others may be identified if they contain compounds that fluoresce when exposed to ultraviolet light. Many zones may also be rendered visible by spraying the plate with certain reagent dyes or stains. The adsorbent may also be scraped off various regions of the plate and the separated molecules eluted from the adsorbent particles. Table 13-4 lists some frequently used adsorbents and their applications.
Ion-Exchange Chromatography:
Proteins and other macromolecules may also be separated by the technique known as ion-exchange chromatography. The separation is usually carried out in tall glass columns packed with grains of a synthetic ion-exchange resin (polymers to which numerous ionizable groups have been chemically added). Resins bearing negative charges are called cation exchangers and positively charged resins are anion exchangers. As a solution of ions is passed through the column, the ions compete with each other for the charged sites on the resin.
Consequently, the rate of movement of any ion through the column depends on its affinity for the resin sites, its degree of ionization, and the nature and concentration of competing ions in the solution. The differential rates of movement of ions through the column are the basis for protein and nucleic acid separations, because these molecules possess a variety of positively and negatively charged groups.
Some ion exchangers used for protein and nucleic acid separations are listed in Table 13-5. Among the resins most widely used for protein separations are diethylaminoethylcellulose (DEAE-cellulose, an anion exchanger) and carboxymethylcellulose (CM-cellulose, a cation exchanger) developed by E. A. Peterson and H. A. Sober.
It is generally believed that the interaction between a resin and a protein involves the formation of electrostatic bonds between the charged sites on the resin particle and oppositely charged dissociated side chains of certain amino acids. Because a number of bonds are formed, proteins are more firmly bound than singly charged substances. For example, at the appropriate alkaline pH, DEAE-cellulose could form bonds with the negatively charged side chains of aspartic acid, glutamic acid, and tyrosine residues present in a protein.
This also implies that the affinity of a protein for the resin can be altered a change in pH. A reduction in pH sequentially suppresses the formation of bonds between the protein and DEAE- cellulose as hydrogen ions bind to the negative side chains of the protein and displace the resin. A similar result would be effected by the addition of silt ions, which would compete with proteins for the resin sites (Fig 3-19).
The separation of proteins by ion-exchange chromatography is carried out as follows. The resin is suspended in a buffer or salt solution (called the starting solvent) and the resulting slurry is used to fill the chromatographic column. At this time, the charges on the resin are neutralized by ions in the solvent.
The sample (also dissolved in the starting solvent) is applied at the top of the column as a narrow zone. Depending on the pH and salt concentration of the solvent, certain proteins present in the sample will form electrostatic bonds with sites on the resin (displacing solvent ions formerly bound at those sites) and the remaining proteins remain in the solvent.
If a volume of the starting solvent is now passed through the column, the unbound proteins will be carried away with the flow and elute at the base of the column. To displace other proteins from the sample zone, it is necessary to change to another solvent whose pH or ionic strength will alter the degree of ionization of the amino acid side chains or compete more effectively with the proteins for the charged sites of the resin.
In the case of proteins adsorbed to DEAE-cellulose or other anion exchangers, this can be accomplished by decreasing the pH and increasing the salt concentration of the solvent. If the second solvent differs only slightly from the first, only a small group of proteins will be released from the sample zone.
During their descent through the column, transient bonds will be formed with the resin many times as the proteins and solvent ions compete for the resin sites. Depending on the relative affinities of the proteins for the resin and the solvent ions, different proteins may reach the bottom of the column at different times.
Thus, changing the solvent not only releases an additional group of proteins but also separates them into a series of zones during their passage through the column. The sequential addition of a series of solvents of different pH and salt concentration, called stepwise elution, eventually displaces and separates all the proteins in the sample zone.
Alternatively, the proteins may be eluted by passing a solvent of continuously changing pH and ionic strength through the column—a method known as gradient elution. The effluent from the column is collected as a series of fractions (Fig. 13-20).
The conditions required for protein desorption depend on the number of bonds formed between the protein and resin and on the nature of the ionized amine acid side chains. Therefore, two proteins having the same net surface charge density (and, consequently, similar electrophoretic mobilities) might be desorbed under quite different conditions and emerge from the column at different times if their absolute surface charges differ.
For example, under a given set of conditions, one protein may bear eight negative and five positive amino acid side chains and have a net charge of – 3; another may have four negative and one positive side chain and be more weakly bound to the resin (desorbing earlier) but would have a similar electrophoretic mobility (i.e., its net charge would also be – 3). For the same reason, two proteins desorbed under the same conditions and emerging from the column together may have quite different electrophoretic mobilities. Consequently, the re-chromatography of a given protein fraction or its further examination by electrophoresis is usually recommended to evaluate the purity of isolated components.
Although the discussion of ion-exchange chromatography has centered around protein separations, it should be recalled that mixtures of different nucleic acids may also be resolved using this technique. Polyysine-kieselguhr columns have been particularly successful for chromatographic separations of both ribonucleic acids and deoxyribonucleic acids.
Affinity Chromatography:
Affinity chromatography is a novel form of column chromatography in which the molecules (principally proteins and nucleic acids) to be isolated from the sample under study are retarded in their passage through the column by their specific biological reaction with the column matrix. It is the biological nature of the interaction between the sample and the column matrix that distinguishes this form of chromatography from others.
The specificity may take several forms, including:
(1) An antigen-antibody reaction,
(2) The interaction of an enzyme with its substrate, allosteric effector, or coenzyme, and
(3) Hydrogen bonding between complementary polynucleotides.
The column is packed with porous gel particles (usually agarose, cross-linked dextrans, or cellulose) to which ligands having a high affinity for specific biological components have been covalently coupled. When the sample is applied to the column, only the constituents having a high affinity for the ligand are bound and other components are rapidly eluted. The ultimate desorption and isolation of the bound species are achieved by significantly altering the pH and/or ionic strength of the eluent (Fig. 13-21).
Affinity chromatography has been used with great success for the isolation of specific immunoglobulin’s from antisera. This is achieved by first coupling the gel with specific antigenic materials. When antiserum is applied to the column, specific immunoglobulin’s react with the antigen-coated gel particles and are retained by the column and other species are eluted.
Gels to which the hormone insulin has been covalently bound have been used to isolate insulin-binding receptor sites of cell membranes. Specific cellular enzymes may be isolated by passing a tissue extract through a column in which the gel particles have previously been derivitized with the enzyme’s substrate or specific co- factor or competitive inhibitor.
Messenger RNA can be isolated from tissue homogenates using agarose impregnated with polyuridylic acid (polyU); mRNA’s affinity for the column matrix results from the occurrence of sequences of polyA in many eukaryotic mRNAs.
Gel Filtration:
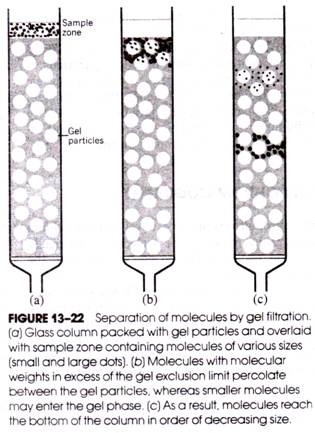
In 1959, J. Porath and P. Flodin introduced gel filtration, a chromatographic method for separating molecules on the basis of size (i.e., molecular weight) differences; the technique is also popularly known as exclusion chromatography, gel permeation chromatography, and molecular sieving.
The procedure is carried out using glass columns packed with nonionic, porous gel particles. Because the gels do not possess ionic groups, separation of a mixture of macromolecules does not involve the temporary formation of bonds with the gel.
The most commonly used gels are cross-linked dextrans (produced by reacting dextran with epichlorohydrin or acrylamide) in which the degree of cross-linkage determines the average pore size of the gel. Gel particles may be produced with a variety of pore sizes.
In gel filtration, the dry gel particles are first swollen by hydration in water or buffer and then packed into a glass column. The volume of the column is effectively divided into two phases: the solvent surrounding the gel particles (solvent phase) and the solvent within the gel particles (gel phase).
Whether or not a given solute molecule can pass between phases depends on the molecular weight of the solute and on the pore sizes of the gel. Any solute molecules larger than the largest pore size cannot pass into the gel phase and are said to be above the exclusion limit of the gel. Smaller molecules are able to penetrate the gel phase to varying degrees.
If a mixture of molecules of various sizes is placed at the top of the column and is followed by the passage of solvent through the column, molecules above the gel exclusion limit will pass between the gel particles and emerge from the bottom of the column most rapidly. Molecules below the exclusion limit will pass through both the solvent and gel phases and emerge later.
Therefore, molecules in the original sample are quickly separated into two different populations- molecules above and those below the gel exclusion limit (Fig. 13-22). However, if two molecules are below the gel exclusion limit but one is larger than the other, the larger molecule will pass through the column more rapidly, because it will spend less time in the gel phase than the smaller molecule (i.e., the chances that a sufficiently large gel pore will be encountered during descent through the column are less for larger molecules below the exclusion limit than for smaller molecules). Hence, even molecules below the gel exclusion limit undergo a separation and emerge from the column in order of decreasing size.
Cross-linked dextran and agarose gels may be obtained with a variety of exclusion limits and are used routinely for protein, nucleic acid, polysaccharide, and lipid separations. In most cases, a single solvent may be used to effect the separation, as molecules are not bound to the gel. In addition to its usefulness for separating different size macromolecules, gel filtration is also used to estimate molecular weights. As in ion-exchange chromatography and electrophoresis, the determination of the purity of separated components may require additional analyses, because different molecules may have the same size and pass through the column at the same rate.
HPLC:
HPLC (high-performance liquid chromatography) is a specialized and versatile form of chromatography that, depending on the particular combination of packing material and solvent, can incorporate the principles of partition, ion-exchange, exclusion, or affinity chromatography. HPLC columns are generally shorter (e.g., 10-20 cm) and much narrower (e.g., 1-5 mm) and are packed with much smaller particles of the adsorbent, resin, or gel in which the separation of the sample is to be effected.
The particles comprising the column packing material rarely exceed 5-10 nm in diameter and form a tightly packed bed that offers high resistance to the flow of solvent. To withstand the very high pressures (e.g., 5000-8000 psi) that must be used to pump the solvent through the column, the columns themselves are fabricated from stainless steel. To optimize resolution, the sample must be delivered to the column as a narrow band. This is usually achieved by placing the sample in a microsyringe and quickly injecting it directly into the column through an injection port.
Gas Chromatography:
Gas chromatography is a special form of column chromatography in which a gas is used as the mobile phase (instead of a liquid) and either a liquid or a solid is used as the stationary phase. When a liquid is used as the stationary phase, the technique is called gas- liquid chromatography (GLC) and separations are based primarily on differences in the partition of the molecules in the sample between the stationary liquid and the moving gas.
In gas-solid chromatography (GSC), separations result from the differential adsorption of sample molecules to the stationary solid phase as they are carried through the column by the gas. Of the two methods, GLC is, by far, the method most often employed.
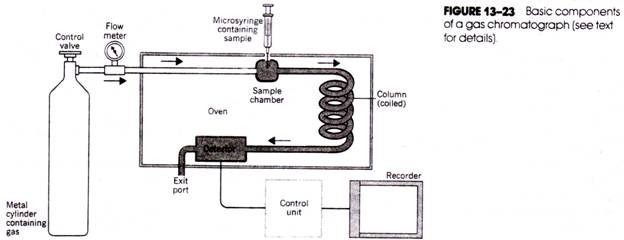
The basic components of the gas chromatograph are the source of gas, the sample introduction chamber, the chromatographic column, the detector, and the recorder (Fig. 13-23). The gas (usually nitrogen, carbon dioxide, helium, or argon) is contained within a high- pressure cylinder connected to the column through metal tubing. A valve, pressure gauge, and flow meter are used to accurately regulate the flow of gas. The sample is introduced into the flow of gas using a micro syringe and needle inserted through a self- sealing diaphragm in the sample chamber.
The chamber itself is enclosed within a heating block so that the sample (if it is not already in a gaseous form) will immediately be vaporized on introduction into the chamber and will be swept into the column by the gas. Gas chromatograph columns are made of glass, copper, or stainless steel tubing and are also enclosed in an oven; because they may be several feet long, they are often twisted to form a spiral.
The selection of packing material for the column depends on whether the separation is to be based on partition or adsorption. For GLC, the column is packed with an inert solid such as kieselguhr, which is impregnated and lightly coated with a liquid of low volatility (so that it will not be eluted from the column at the operating temperature used).
For GSC, the stationary phase is an adsorbent such as charcoal or silica gel. Different molecules in the sample will be carried through the column at different rates, depending on- their adsorption or partition characteristics, and will emerge from the end of the column at different times.
Located near the exit of the column and also housed within an oven is the detector that monitors the composition of the emerging gas and relays electrical signals proportional to the amounts of separated components to a strip-chart recorder.
The separated components are thus recorded as a series of peaks in the chromatogram tracing. The most common form of detector is the flame ionization chamber in which the components are successively mixed with hydrogen and air and burned in a high-voltage field. Migration of the ionized fragments in this field creates a current registered by the recorder.
Most chromatographic separations are analytical and the technique is so sensitive that only minute quantities of sample are required. However, separated components may also be collected as a condensate in tubes as they emerge from the heated column and are rapidly cooled.
Gas chromatography may be used to separate lipids, oligosaccharides, and amino acids after their preliminary conversion to volatile derivatives (many lipids may be chromatographed without conversion). Gas chromatography has been used with great success for the separation of different fatty acids. Depending on whether a nonpolar or a polar liquid phase is used, fatty acids may be separated according to boiling point and size or degree of saturation.