ADVERTISEMENTS:
This article throws light upon the top six types of tools of biochemistry.
The top six types of tools of biochemistry are: (1) Chromatography (2) Electrophoresis (3) Photometry—Colorimeter and Spectrophotometer (4) Ultracentrifugation (5) Radioimmunoassay and (6) Enzyme-Linked Immunosorbant Assay.
The foundations for the present (and the future, of course) knowledge of biochemistry are based on the laboratory tools employed for biochemical experimentation.
ADVERTISEMENTS:
The basic principles of some of the commonly employed tools are described here:
Tool # 1. Chromatography:
Chromatography is one of the most useful and popular tools of biochemistry. It is an analytical technique dealing with the separation of closely related compounds from a mixture. These include proteins, peptides, amino acids, lipids, carbohydrates, vitamins and drugs.
Principles and Classification:
Chromatography (Greek: chroma — colour; graphein — to write), usually consists of a mobile phase and a stationary phase. The mobile phase refers to the mixture of substances (to be separated), dissolved in a liquid or a gas. The stationary phase is a porous solid matrix through which the sample contained in the mobile phase percolates.
The interaction between the mobile and stationary phases results in the separation of the compounds from the mixture. These interactions include the physicochemical principles such as adsorption, partition, ion-exchange, molecular sieving and affinity.
ADVERTISEMENTS:
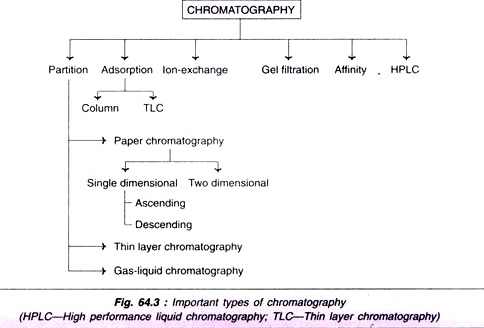
The interaction between stationary phase and mobile phase is often employed in the classification chromatography e.g. partition, adsorption, ion- exchange. Further, the classification of chromatography is also based either on the nature of the stationary phase (paper, thin layer, column), or on the nature of both mobile and stationary phases (gas-liquid chromatography). A summary of the different methods (classes) of chromatography is given in Fig. 64.3.
1. Partition chromatography:
The molecules of a mixture get partitioned between the stationary phase and mobile phase depending on their relative affinity to each one of the phases.
2. Adsorption column chromatography:
The adsorbents such as silica gel, alumina, charcoal powder and calcium hydroxyapatite are packed into a column in a glass tube. This serves as the stationary phase. The sample mixture in a solvent is loaded on this column. The individual components get differentially adsorbed on to the adsorbent.
The elution is carried out by a buffer system (mobile phase). The individual compounds come out of the column at different rates which may be separately collected and identified. For instance, amino acids can be identified by ninhydrin colorimetric method. An automated column chromatography apparatus — fraction collector—is frequently used nowadays.
3. High performance liquid chromatography (HPLC):
In general, the chromatographic techniques are slow and time consuming. The separation can be greatly improved by applying high pressure in the range of 5,000-10,000 psi (pounds per square inch), hence this technique is also referred (less frequently) to as high pressure liquid chromatography.
ADVERTISEMENTS:
More information on chromatography with special reference to gel-filtration chromatography, ion- exchange chromatography, affinity chromatography and hydrophobic interaction chromatography is given under downstream processing.
Tool # 2. Electrophoresis:
The movement of charged particles (ions) in an electric field resulting in their migration towards the oppositely charged electrode is known as electrophoresis. Molecules with a net positive charge (cations) move towards the negative cathode while those with net negative charge (anions) migrate towards positive anode. Electrophoresis is a widely used analytical technique for the separation of biological molecules such as plasma proteins, lipoproteins and immunoglobulin’s.
Different Types of Electrophoresis:
1. Zone electrophoresis:
An inert supporting material such as paper or gel are used.
ADVERTISEMENTS:
2. Isoelectric focusing:
This technique is primarily based on the immobilization of the molecules at isoelectric pH during electrophoresis. Stable pH gradients are set up (usually in a gel) covering the pH range to include the isoelectric points of the components in a mixture. Isoelectric focusing can be conveniently used for the purification of proteins.
3. Immunoelectrophoresis:
This technique involves combination of the principles of electrophoresis and immunological reactions. Immunoelectrophoresis is useful for the analysis of complex mixtures of antigens and antibodies.
Tool # 3. Photometry—Colorimeter and Spectrophotometer:
Photometry broadly deals with the study of the phenomenon of light absorption by molecules in solution. The specificity of a compound to absorb light at a particular wavelength (monochromatic light) is exploited in the laboratory for quantitative measurements.
ADVERTISEMENTS:
From the biochemist’s perspective, photometry forms an important laboratory tool for accurate estimation of a wide variety of compounds in biological samples. Colorimeter and spectrophotometer are the laboratory instruments used for this purpose. They work on the principles discussed below.
When a light at a particular wavelength is passed through a solution (incident light), some amount of it is absorbed and, therefore, the light that comes out (transmitted light) is diminished. The nature of light absorption in a solution is governed by Beer-Lambert law.
Beer’s law states that the amount of transmitted light decreases exponentially with an increase in the concentration of absorbing material (i.e. the amount of light absorbed depends on the concentration of the absorbing molecules). And according to Lambert’s law, the transmitted light decreases exponentially with increase in the thickness of the absorbing molecules (i.e. the amount of light absorbed is dependent on the thickness of the medium).
ADVERTISEMENTS:
The ratio of transmitted light (I) to that of incident light (IO) is referred to as transmittance (T).
Absorbance (A) or optical density (OD) is very commonly used in laboratories. The relation between absorbance and transmittance is expressed by the following equation.
A = 2 – log10T
= 2 – log% T
Colorimeter:
Colorimeter (or photoelectric colorimeter) is the instrument used for the measurement of coloured substances. This instrument is operative in the visible range (400-800 nm) of the electromagnetic spectrum of light. The working of colorimeter is based on the principle of Beer-Lambert law.
ADVERTISEMENTS:
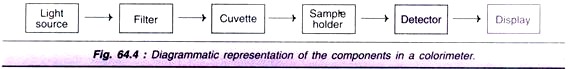
The colorimeter, in general consists of light source, filter sample holder and detector with display (meter or digital). A filament lamp usually serves as a light source. The filters allow the passage of a small range of wave length as incident light.
The sample holder is a special glass cuvette with a fixed thickness. The photoelectric selenium cells are the most common detectors used in colorimeter. The diagrammatic representation of a colorimeter is depicted in Fig. 64.4.
Spectrophotometer:
The spectrophotometer primarily differs from colorimeter by covering the ultraviolet region (200- 400 nm) of the electromagnetic spectrum. Further, the spectrophotometer is more sophisticated with several additional devices that ultimately increase the sensitivity of its operation several fold when compared to a colorimeter.
A precisely selected wavelength (say 234 nm or 610 nm) in both ultra violet and visible range can be used for measurements. In place of glass cuvettes (in colorimeter), quartz cells are used in a spectrophotometer. The spectrophotometer has similar basic components as described for a colorimeter (Fig. 64.4) and its operation is also based on the Beer-Lambert law.
Tool # 4. Ultracentrifugation:
Ultracentrifugation is an indispensable tool for the isolation of subcellular organelles, proteins and nucleic acids. In addition, this technique is also employed for the determination of molecular weights of macromolecules. The rate at which the sedimentation occurs in ultracentrifugation primarily depends on the size and shape of the particles or macromolecules (i.e. on the molecular weight). It is expressed in terms of sedimentation coefficients).
ADVERTISEMENTS:
The sedimentation coefficient has the units of seconds. It was usually expressed in units of 10-13 s (since several biological macromolecules occur in this range), which is designated as one Svedberg unit. For instance, the sedimentation coefficient of hemoglobin is 4 × 10-13 s or 4S; ribonuclease is 2 × 10-13 s or 2S. Conventionally, the subcellular organelles are often referred to by their S value e.g. 70S ribosome.
Isolation of Subcellular Organelles by Centrifugation:
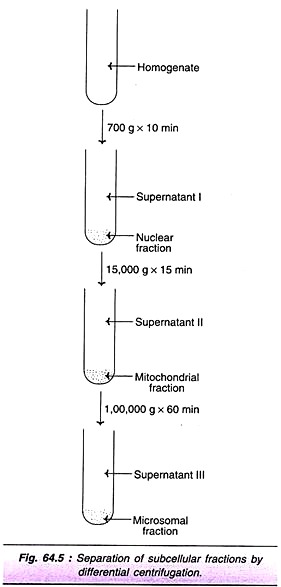
The cells are subjected to disruption by sonication or osmotic shock or by use of homogenizer. This is usually carried out in an isotonic (0.25 M) sucrose. The advantage with sucrose medium is that it does not cause the organelles to swell. The subcellular particles can be separated by differential centrifugation. The most commonly employed laboratory method separates subcellular organelles into 3 major fractions— nuclear, mitochondrial and microsomal (Fig. 64.5).
When the homogenate is centrifuged at 700 g for about 10 min, the nuclear fraction (includes plasma membrane) gets sedimented. On centrifuging the supernatant (I) at 15,000 g for about 5 min mitochondrial fraction (that includes lysosomes, peroxisomes) is pelleted. Further centrifugation of the supernatant (II) at 100,000 g for about 60 min separates microsomal fraction (that includes ribosomes and endoplasmic reticulum). The supernatant (III) then obtained corresponds to the cytosol.
The purity (or contamination) of the subcellular fractionation can be checked by the use of marker enzymes. DNA polymerase is the marker enzyme for nucleus, while glutamate dehydrogenase and glucose 6-phosphatase are the markers for mitochondria and ribosomes, respectively. Hexokinase is the marker enzyme for cytosol.
Tool # 5. Radioimmunoassay:
Radioimmunoassay (RIA) was developed in 1959 by Solomon, Benson and Rosalyn Yalow for the estimation of insulin in human serum. This technique has revolutionized the estimation of several compounds in biological fluids that are found in exceedingly low concentrations (Nano gram or picogram). RIA is a highly sensitive and specific analytical tool.
Principle:
Radioimmunoassay combines the principles of radioactivity of isotopes and immunological reactions of antigen and antibody, hence the name. The principle of RIA is primarily based on the competition between the labeled and unlabeled antigens to bind with antibody to form antigen- antibody complexes (either labelled or unlabeled). The unlabeled antigen is the substance (say insulin) to be determined. The antibody to it is produced by injecting the antigen to a goat or a rabbit.
The specific antibody (Ab) is then subjected to react with unlabeled antigen in the presence of excess amounts of isotopically labeled (131l) antigen (Ag+) with known radioactivity. There occurs a competition between the antigens (Ag+ and Ag) to bind the antibody. Certainly, the labeled Ag+ will have an upper hand due to its excess presence.
As the concentration of unlabeled antigen (Ag) increases the amount of labelled antigen-antibody complex (Ag+-Ab) decreases. Thus, the concentration of Ag+-Ab is inversely related to the concentration of unlabeled Ag i.e. the substance to be determined. This relation is almost linear.
A standard curve can be drawn by using different concentrations of unlabeled antigen and the same quantities of antibody and labeled antigen. The labeled antigen-antibody (Ag+-Ab) complex is separated by precipitation. The radioactivity of 131 present is Ag+-Ab is determined.
Applications:
RIA is no more limited to estimating of hormones and proteins that exhibit antigenic properties. By the use of haptens (small molecules such as dinitrophenol, which, by themselves, are not antigenic), several substances can be made antigenic to elicit specific antibody responses.
In this way, a wide variety of compounds have been brought under the net of RIA estimation. These include peptides, steroid hormones, vitamins, drugs, antibiotics, nucleic acids, structural proteins and hormone receptor proteins. Radioimmunoassay has tremendous application in the diagnosis of hormonal disorders, cancers and therapeutic monitoring of drugs, besides being useful in biomedical research.
Tool # 6. Enzyme-Linked Immunosorbant Assay:
Enzyme-linked immunosorbant assay (ELISA) is a non-isotopic immunoassay. An enzyme is used as a label in ELISA in place of radioactive isotope employed in RIA. ELISA is as sensitive as or even more sensitive than RIA. In addition, there is no risk of radiation hazards (as is the case with RIA) in ELISA.
Principle:
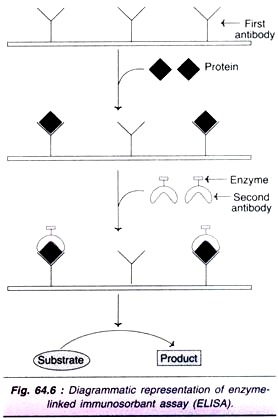
ELISA is based on the immunochemical principles of antigen-antibody reaction. The stages of ELISA, depicted in Fig. 64.6, are summarized.
1. The antibody against the protein to be determined is fixed on an inert solid such as polystyrene.
2. The biological sample containing the protein to be estimated is applied on the antibody coated surface.
3. The protein antibody complex is then reacted with a second protein specific antibody to which an enzyme is covalently linked. These enzymes must be easily assayable and produce preferably coloured products. Peroxidase, amylase and alkaline phosphatase are commonly used.
4. After washing the unbound antibody linked enzyme, the enzyme bound to the second antibody complex is assayed.
5. The enzyme activity is determined by its action on a substrate to form a product (usually coloured). This is related to the concentration of the protein being estimated.
Applications:
ELISA is widely used for the determination of small quantities of proteins (hormones, antigens, antibodies) and other biological substances. The most commonly used pregnancy test for the detection of human chorionic gonadotropin (hCG) in urine is based on ELISA. By this test, pregnancy can be detected within few days after conception. ELISA is also useful for the diagnosis of AIDS.