ADVERTISEMENTS:
The below mentioned article provides a short note on the Disorders of Plasma Membranes.
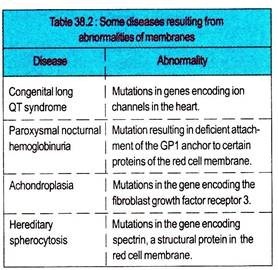
Mutations affecting the protein constituents of membranes can cause many diseases. Proteins in membranes are classified as receptors, transporters, ion channels, enzymes, and structural components. All these classes are often glycosylated, so that mutations affecting this process may change their function.
Almost 30 genetic disorders have been attributed to mutations affecting various proteins involved in the transport of amino acids, sugars, lipids, urate, anions, cations, water, and vitamins across the plasma membrane.
ADVERTISEMENTS:
Mutations in genes encoding mitochondrial membrane proteins involved in oxidative phosphorylation can cause neurological and other problems. Membrane proteins are also affected by conditions other than mutations.
Myasthenia gravis is caused by the formation of autoantibodies to the acetylcholine receptor in skeletal muscle. Ischemia can promptly affect the integrity of various ion channel in membranes. Abnormalities of membrane constituents other than protein are also harmful.
Excess of cholesterol (e.g., in familial hypercholesterolemia), excess of lysophospholipid (e.g., the venom after biting of certain snakes contains phospholipases), or excess of glycosphingolipids (e.g., in a sphingolipidosis) can affect membrane function.
Multiple Sclerosis:
ADVERTISEMENTS:
It is a somewhat confusing neurologic disorder in which nervous tissue is demyelinated by an unknown process. The demyelinization removes neuronal insulation and profoundly decreases the velocity of nerve impulse transmission.
Pseudohypoparathyroidism:
It is an inherited disorder which signifies defective signalling across the plasma membranes of several types of target cells. In that affected cell, the parathormone signal is normally mediated by activation of adenylate cyclase. There is a defective coupling protein in the membrane that prevents transmission of the extracellular hormone signal to the adenylate cyclase.
Cell Disease:
It is a recessive disorder due to inability of the patient’s fibroblasts to attach the mannose-6-phosphate signal to the acid hydrolases synthesized in various cell types. The acid hydrolases do not segregate normally to the primary lysomes but are secreted into the extracellular space leaving the intracellular lysosomes deficient in these important hydrolases.
Thus, the lysosomes accumulate excessive debris and thereby generate intracellular cytoplasmic inclusions (I). These abnormal secreted hydrolases are not subject to adsorptive pinocytosis by the patient’s or even by normal fibroblasts, since they lack the necessary mannose-6-phosphate recognition signal.
Each chain is divided into specific domains or regions that have structural and functional significance. The half of the light chain (L) toward the carboxy terminus is termed as the constant region (CL), while the amino terminal half is the variable region (VL) of the light chain.
ADVERTISEMENTS:
About one quarter of the heavy (H) chain at the amino terminus is termed as variable region (VH), and the other three quarters of the heavy chain are referred to as the constant regions (CH1, CH2, CH3) of the H chain.
The portion of the immunoglobulin molecule which binds the specific antigen is formed by the amino-terminal portions (variable regions) of both the H and L chains, i.e., the VH and VL domains. The domains of the protein chains do not simply exist as linear sequences of amino acids but form globular regions with secondary and tertiary structure.