ADVERTISEMENTS:
In this article we will discuss about the process of oxidation of fatty acids.
Oxidation of Even-Carbon, Saturated Straight Chained Fatty Acids:
A. Activation of Fatty Acids:
Before they are oxidized, fatty acids must be activated. This activation requires energy (supplied by ATP) and takes place in 2 steps, as may be seen in figure 5-10.
In a first step, ATP reacts with the fatty acid to form a mixed anhydride (with liberation of pyrophosphate). This step is very similar to the reaction of activation of amino acids on the biosynthesis of proteins. Then, in a second step, the acyl- AMP formed reacts with coenzyme A-SH to give a thio-ester, the acyl-coenzyme A (with liberation of AMP).
B. Reactions of β-Oxidation:
ADVERTISEMENTS:
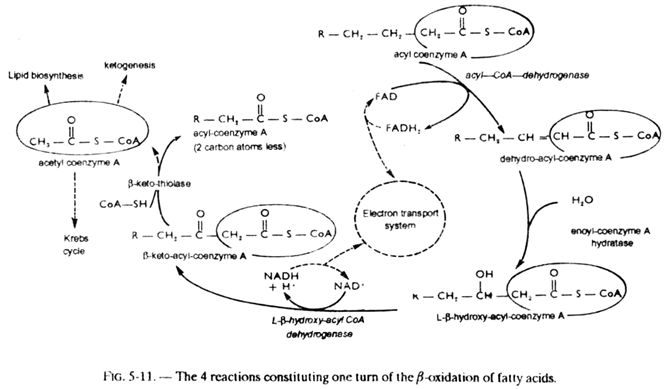
The acyl-coenzyme A thus formed can then follow the pathway of β-oxidation which comprises the following reactions (see fig. 5-11).
1. Dehydrogenation under the action of an acyl-coA-dehydrogenase, a FAD enzyme, with formation of a α-β double bond in trans configuration;
ADVERTISEMENTS:
2. Hydration of this double bond, catalyzed by enoyl-coA hydratase (crotonase), with formation of α β-hydroxylated derivative of L-configuration;
3. Dehydrogenation by L-β-hydroxyacyl-coA -dehydrogenase, a NAD+ enzyme, with formation of a β-keto derivative;
4. By intervention of a molecule of coenzyme A, detachment of a two carbon fragment in the form of acetyl-coA, catalyzed by β-ketothiolase. This is thiolysis or “thioclastic scission.” There remains an acyl-coA having 2 carbon atoms less than the starting acyl-coA (or fatty acid).
This set of 4 reactions can be summarized as follows:
CH3(CH2)n—CO—S—coA + FAD + NAD+ + coA—SH → CH3 —(CH2)n-2—CO—S—coA + FADH2 + NADH + H+ + acetyl— coA
Then the acyl-coA formed (having 2 carbon atoms less than the starting fatty acid) will, in its turn, undergo the same series of 4 reactions to give an acetyl- coA and a new acyl-coA (having 4 carbon atoms less than the starting fatty acid) and so on. As proposed by Lynen, β-oxidation may be represented by a helix, each turn of which corresponds to a shortening of the fatty acid by 2 carbon atoms (liberated in the form of acetyl-coA) and a departure of 4H (see fig. 5-13).
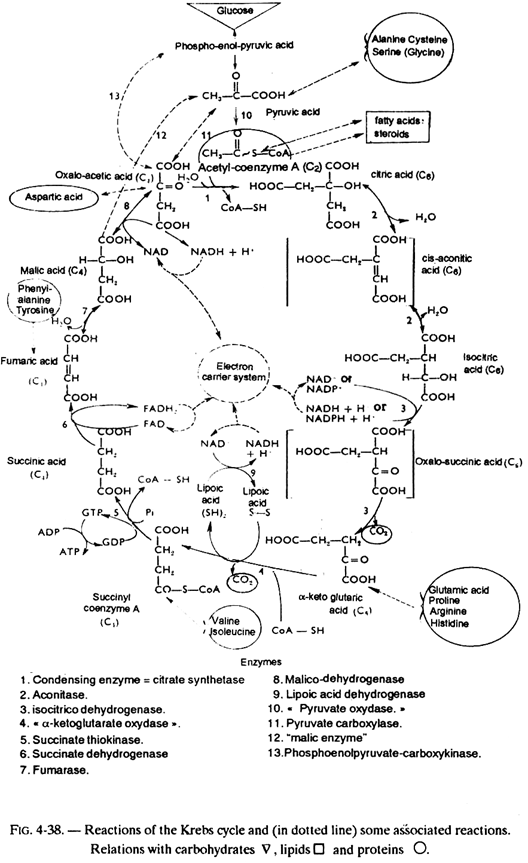
The β-oxidation of fatty acids in an intra-mitochondrial process. The molecules of acetyl-coA formed will be used either in anabolic processes (biosynthesis of fatty acids and isoprenic lipids), or in ketogenesis, or they will be completely oxidized by the Krebs cycle. The reactions of the Krebs cycle is examined in fig. 4-38.
Besides this β-oxidation, there exists a second pathway: the peroxysomal β-oxidation which takes place in the peroxysomes. The reactions are identical to those of the mitochondrial β-oxidation. Only the first enzyme is different. It transfers directly the 2 H atoms removed from the acyl-coenzyme A during the formation of the double bond, to cytochrome a3 with formation of H2C2.
ADVERTISEMENTS:
In plants, peroxysomal β-oxidation is the most important catabolism process of fatty acids. This system also exists in some tissues of mammals like the liver or kidney, and in yeasts, moulds and ciliates.
C. Energy Balance of the β-Oxidation of Fatty Acids:
Each turn of the helix of β-oxidation yields FADH2 and NADH + H+ the reoxidation of which, thanks to the electron transport chain, allows the formation respectively, of 2 and 3 molecules of ATP, i.e. a total of 5 ATP per turn (or per unit of 2 carbon atoms detached from the molecule of fatty acid).
The acetyl-coA is then oxidized by the Krebs cycle with formation of 12 ATP. The complete oxidation of one unit of 2 carbon atoms therefore yields 17 ATP. Referring to the example of stearic acid (see fig. 5-12) which has 18 carbon atoms, one can see that its complete oxidation will allow the formation of (8 x 5) + (9 x 12) i.e. 148 ATP, and after deducting the ATP required for the preliminary activation of the fatty acid, there is a gain of 147 ATP.
The energy yield per carbon atom is therefore of the order of 8 ATP (147/18) in the oxidation of fatty acids and 6 (38/6) in the oxidation of glucose. Moreover, triglycerides contain many more carbon atoms per unit weight than the polysaccharides and can therefore constitute larger energy reserves for a much smaller weight.
At the end, let us point out that the enzyme implied in the β-oxidation of fatty acids are mainly located in the mitochondria, just like the enzymes responsible for electron transport and oxidative phosphorylation.
One can therefore make the same observation as in the case of Krebs cycle (which is also an intra-mitochondrial process): it appears that in the mitochondria there is a concentration of enzymatic systems which permits on the one hand, the oxidation of the energy reserve substances, and on the other hand, the formation of ATP, which must enhance the efficiency of these energy transfers.
However, due to intra-mitochondrial location of β-oxidation, the acetyl- coenzyme A which is required for the synthesis of fatty acids or sterols, has to leave the mitochondrion. It comes out in the form of citrate formed by reaction of acetyl-coenzyme A with the oxalacetate.
ADVERTISEMENTS:
In the cytoplasm, citric acid is cleaved by ATP-citrate lyase which, in presence of a molecule of coenzyme A and ATP, yields one molecule of acetyl-coenzyme A and one molecule of oxalacetate (ATP being hydrolyzed to ADP + Pi).
Oxidation of Odd-Carbon Atoms Saturated Fatty Acids:
They also undergo β-oxidation, but the 5-carbon atom acyl-coenzyme A, obtained at the end of the last but one turn of the helix (instead of butyryl co-A, see fig. 5-12) is cleaved to acetyl-coenzyme A + propionyl-coenzyme A.
The latter undergoes a carboxylation (in presence of propionyl-coA-carboxylase, biotin and ATP) and then isomerization (in presence of methyl malonyl-coA mutase which has a cobamide type coenzyme, derived from vitamin B12), yielding succinyl-coenzyme A, an intermediate of the Krebs cycle, (see fig. 5-13).
Oxidation of Unsaturated Fatty Acids:
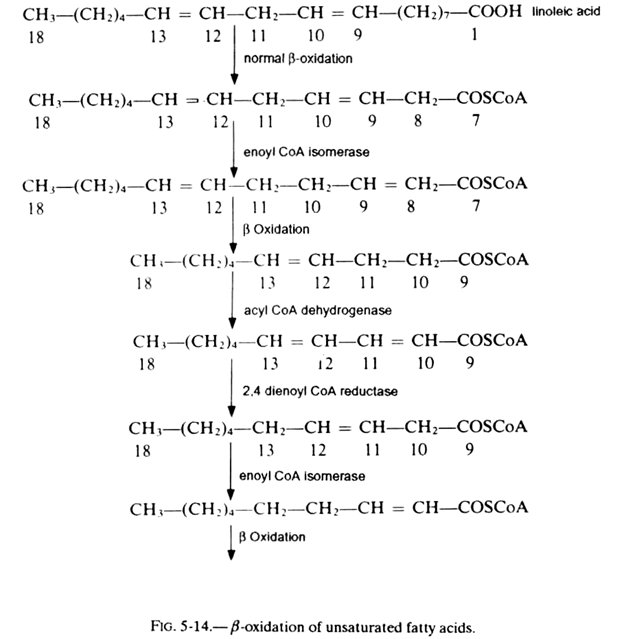
The catabolism of unsaturated fatty acids takes place by a modification of the β-oxidation consecutive to the position of double bonds. We shall take the example of linoleic acid which is the most complex case (see fig. 5-15).
ADVERTISEMENTS:
The numbering of carbon atoms is indicated below each formula:
The reaction begins with a conventional β-oxidation till formation of an acyl coA with 12 carbon atoms having a double bond cis in β-γ. Thanks to an isomerase, the latter is converted into a double bond trans placed in α-β. The acyl coA obtained will therefore continue to be catabolized by the conventional reactions of β-oxidation yielding a fatty acid with 10 carbon atoms.
Acyl coA dehydrogenase introduces a double bond. After the action of 2, 4 dienoyl coA reductase which has NADPH as coenzyme, the 2 double bonds are replaced by a single one localized in β-y. The intervention of enoyl coA isomerase permits the introduction of this latter compound into the normal cycle of β-oxidation.
In the case of a fatty acid like oleic acid (18:1(9)), only the first 3 reactions of figure 5-14 come into play. This catabolism pathway is present in mitochondria and peroxysomes of the liver. It was found in mammals. An analogous system exists in fungi and bacteria.
Oxidation of Branched Fatty Acids:
We will take the example of a-methyl-butyryl-coenzyme A, formed from isoleucine (glucoformer and ketoformer aminoacid) by deamination or transamination (reactions that we will study in connection with the metabolism of amino acids), then oxidative decarboxylation.
ADVERTISEMENTS:
As may be seen in figure 5-15, the presence of the methyl group does not prevent the usual reactions of β-oxidation, and one observes the successive formation of the α-β-unsaturated derivative, β-hydroxylated derivative and β-keto derivative.
Thiolysis finally gives:
1. Acetyl-coA which can be used for the synthesis of various lipids or for ketogenesis (hence the ketoformer character of isoleucine);
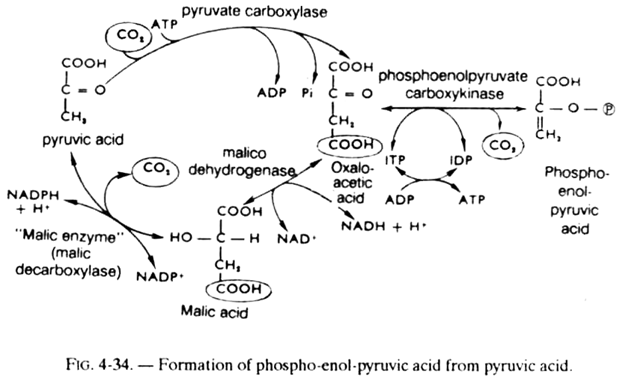
2. Propionyl-coA which will be converted, as just seen above (see fig. 5-13), into succinyl-coA, an intermediate of the Krebs cycle which will be transformed (within the cycle) into oxaloacetic acid. The latter can be converted into phospho-enol-pyruvic acid (see fig. 4-34), then into glucose by the neoglucogenesis reactions, which explains the glucoformer character of isoleucine.