ADVERTISEMENTS:
The following points highlight the top three types of chromatography techniques. The chromatography techniques are: 1. Paper Chromatography 2. Thin Layer Chromatography and 3. Column Chromatography.
Chromatography Technique # 1. Paper Chromatography:
Paper chromatography is useful for separating the mixture of amino acids, sugars, chemicals, lipids, urea and some drugs. It is used to separate and identify all sorts of substances in forensics. Narcotic drugs and aspirin can be identified in blood and urine samples. It is useful in separating complex mixture of similar compounds e.g. amino acids.
Paper chromatography is a partition chromatography technique. Cellulose paper is a supporting medium over which the solvents flow. Water bound to the polar cellulose is the stationary phase and organic solvent which flows over it is a mobile phase. Organic solvent moves over the hydrated cellulose fibres.
ADVERTISEMENTS:
As the solvent passes through an area of paper containing a solute (mixture of components), the solute begins to partition itself between the aqueous and organic phases in proportion to its relative solubility in the two phases. The components of the solute more soluble in organic phase will be carried faster along the organic phase.
Conversely, greater the affinity for water, slower the solute will move with respect to the solvent front. Thus if several compounds possess different solubility rates, each will move across the paper at specific rate which is generally different from that of any other compound.
The distance the solute moves, in relation to the distance the solvent moves, serves as a means of identifying the solute and is called Rf.
Paper Used:
Pure cellulose paper, Whatman No 1 and No. 3 paper is generally used Modified cellulose paper like DEAE cellulose, CM cellulose and Resin impregnated papers Amberlite are also used. The paper is made up of cellulose fibres and cellulose is a polymer of simple sugar glucose. The polymer chain has -OH groups sticking out around them.
Solvent:
ADVERTISEMENTS:
The choice of solvent depends upon the mixture to be investigated.
Solvent system for sugars generally used is:
Sample Application:
A drop of sample containing the mixture to be separated is applied on the paper as a small spot about 3 cm from one end of the paper strip by means of platinum wire loop or capillary tube or micropipette. The lower and of the paper below the sample is dipped in the solvent.
Irrigation Mode:
There are two techniques:
Ascending Method
Descending Method
In ascending technique, solvent and solute move against gravity vertically upward by capillary action. In descending method solvent and solute move in the direction of gravity.
ADVERTISEMENTS:
A third method called radial development technique. Here the sample mixture is applied at the centre of the paper and it moves outwards radically forming concentric circles of increasing diameter.
Ascending methods gives best results. It takes 40-90 hours for complete run of the solvent.
Identifying the Compounds:
The sample compounds are identified easily if the chromatogram is coloured. If it is colourless, the paper is sprayed with suitable colour producing agents like ninhydrin, Silver nitrate-acetone solution, sodium thiosulphate solution etc. Other methods of detection are by fluorescence, radioactivity, etc.
Paper Chromatogram:
The paper is suspended in a container whose lower end is dipped in the solvent. Mixture is applied on a spot above the solvent level. As the solvent slowly moves up the paper the different compounds of the mixture are carried upwards with it at different rates. In this way components of the mixture are separated into different spots on the paper.
Rf Values:
The distance travelled by a particular compound is constant for the same type of paper used and same type of solvent used. The distance travelled by a compound relative to the solvent is called Rf value.
For each compound it is calculated as follows:
For example, if one component of a mixture travelled 9 cm from the base line while the solvent travelled 12 cm, then the Rf value for that compound is
If the two spots travelled the same distance on the paper, they are most likely to be the same compound but not always as the two different compounds may have the same Rf value.
The position of the solvent fount is marked with pencil before the paper dries up. If the spots on the chromatogram are colourless as in the case of amino acids, then the chromotogram is sprayed with ninhydrin which gives amino acids brown or purple colour. Other colouring agents are silver nitrate-acetone solution, sodium thiosulptate solution. Other methods of detection are fluorescence, redioactivity etc.
Two Way Chromatography:
If two different substances have similar Rf value, they can be separated by the use of two way chromatography. A chromatograph is prepared starting from a single spot. The position of the solved front in marked with pencil as SF1.
When the paper is dried up completely, it is rotated by 90° and chromatograph is developed again with a different solvent. The position of the second solvent front is marked SF2. As can be seen here the spot No. 2 in the second chromatogrpah has been separated into two separate compounds using different solvents though they have same Rf values in the first chromatogram. Therefore the mixture having the similar Rt value can be separated by this technique.
ADVERTISEMENTS:
Rf value of each of the compounds (spot) can be worked out and by comparing these with the known Rf value of different compounds, the compounds can be identified.
Chromatography Technique # 2. Thin Layer Chromatography:
Thin layer chromatography is similar to paper chromatography for identification, separation and purification of components of a mixture. Here ascending technique is used like the paper chromatography.
Principle:
The chromatography technique involves the partition of components of a mixture to be separated between the two phases which move with respect to each other. The two phases are a fixed phase (solid/liquid) and a mobile phase (liquid). This technique is useful for separation of lipids, amino acids and sugars etc.
Adsorbants:
Silica gel with binder gypsum, Alumina, cellulose powder, polyacrylamide etc.
Procedure:
Preparation of TLC plate (stationary phase): Generally glass plates of 5 x 20 cm and 20 x 20 cm are used. Plates are cleaned with chromic acid, detergents and water and then dried. Silica gel paste (stationary phase) is spread on the plate by means of a glass rod and then heated to remove moisture.
Solvent System (Mobile Phase):
ADVERTISEMENTS:
Choice of the solvent system depends upon the nature of the mixture to be analysed.
For non-polar lipids following solvents are used:
For polar lipids following solvents are used:
About 100 ml of solvent is sufficient and it is put in a solvent chamber.
Application of the Mixture:
ADVERTISEMENTS:
With the help of capillary tube, mixture to be analysed is applied as a spot near one end of the plate.
Development of the Plate:
The plate is then dipped in the solvent chamber and the spot containing the mixture slowly starts moving over the plate and reaches near the top.
Marking of Spots:
Majority of the substances are not visible on the plate. For this purpose staining agents like iodine or 50% analar grade sulphuric acid or minhydrin is sprayed on the plate. A fluorescent compound is added to the adsortant that glows in ultraviolet light for easy identification.
Ninhydrin reacts with amino acids to give brown or purple colour. Iodine crystals added release iodine vapours which make some substances brown. The spots become coloured and are marked on the backside of the plate with the help of marking pen.
Identification:
It can be done by calculating the Rf value of different spots.
Rf value for a compound is constant using the same solvent. Normally a standard experiment can be run along-with the unknown compound to locate its presence. It is used in analysing fatty acids, detection of pesticides in food and water, in forensics, in identification of medicinal plants and their constituents.
Earliest definitive work in the field was done by Izmailar and Schraiber which involved the separation of alkaloids. Though TLC is not very efficient, it has advantages of speed, versatility and simplicity.
Chromatography Technique # 3. Column Chromatography:
To understand the function of a specific protein, it needs to be separated and purified. Each protein has some unique properties which make it easier to separate a specific protein from the mixture of proteins. The unique characteristics include its size, shape and ionic charge etc.
In column chromatography, protein fractions are allowed to pass through glass columns filled with appropriate modified acrylamide or agarose beads. Column chromatography is of two types, Gel Alteration or Gel Permeation chromatography and Ion Exchange charomatography.
By passing proteins through a number of different columns, they are increasingly purified.
(a) Gel Permeation Chromatography:
In this technique separation is based on the size of the molecules, therefore it is also called molecular sieve chromatography.
Swollen gel beads or porous glass beads are set in a column, serve as molecular sieve. A mixture containing molecules of different sizes is poured over the column. The larger sized molecules pass through the spaces between the beads and are collected first. Pores of gel beads or glass beads are smaller, through which the large molecules cannot pass.
On the other hand, small sized molecules enter into beads through the pores and thus are separated from the solvent. They travel down slowly through the beads and are collected in a separate stream. Large molecules are excluded from the beads, therefore this technique is also called Exclusion Chromatography.
Commonly used Gels:
They include cross linked dextrons such as Sephadex, Agarose such as Sepharose, and Bio-gel A, Polyacrylamide such as Bio-gel P.
Applications:
It involves the purification of biological molecules such as proteins, enzymes, hormones, antibodies, nucleic acids, polysachharides and viruses etc.
(b) Ion-Exchange Chromatography:
In this technique, the separation depends upon the attraction between oppositely charged ions. Many biological materials like proteins and amino acids have ionisable groups and may carry net positive or net negative charge.
A number of specially modified beads are available some of which are negatively charged, therefore attract positively charged particles. They are called cation exchangers. Similarly, positively charged resins are called Anion exchangers. Most of these resins are derivatives of polymeric cellulose.
The proteins in a mixture carry net positive or negative charge. The sample protein mixture in a solution is allowed to pass through a column having positively charged beads. Only proteins having a net negative charge (acidic proteins) bind tightly to the beads. Neutral and positively charged proteins (basic proteins) flow through the column and are collected in a beaker.
Now a gradient of increasing concentration of salt (Na+ Cl~) is passed trough this column. At high salt concentration negatively charged salt ions (Cl~) bind to the positively charged beads displacing the negatively charged proteins from the beads. These are then collected in a separate beaker.
Commonly used Ion-exchangers:
Common cation exchangers are carboxymethyl cellulose, sulphopropyl etc. Anionic exchangers are Diethylamino ethyl cellulose, triethylaminethyl.
Applications of Ion-exchange Chromatography:
1. Ion exchange technique was used by Chargaff to determine the base composition of nucleic acids.
2. Ion exchange technique is used extensively in water purification. Water is completely deionised by exchanging hydrogen and hydroxyl ions using anion and cation exchangers. Water purification machines work on this principle.
3. Ion exchange is extensively used in analysis of amino acids. Amino acid composition of a protein can be determined with a machine called amino acid analyser which works on ion exchange principle using a strong cation exchanger.
4. Ion exchange is used for the separation of many vitamins and organic acids.
(c) Adsorption Chromatography:
It is a type of column chromatography. Different substances differ in their adsorption behaviour between a solid stationary phase and a moving solvent which is a gas or a liquid
Separation takes place when one of a two component mixture is more strongly adsorbed then other on Ac solid stationary phase. Adsorption is a surface phenomenon. The degree of separation depends upon the surface area of the adsorbant. The solute molecules which interest more strongly with the adsorbent are retarded more. Less interacting solute moleculesare reurdec less,these travel faster and are separated.
Distribution efficient K of the substance between two phases of the system is
M. Tswett was able to separate different components of the plant pigment chlorophyll by adsorbent chromatography.
The plani pigment chlorophyll extract in petroleum ether (eluent) was poured over the powdered CaCO3 in a vertical column in a glass tube. It is irrigated with alcohol. After sometime coloured bands are developed in the column.
The most strongly adsorbed component forms the topmost band, while the least adsorbed component forms the lowermost band. The bands in between are formed by components of the descending order of adsorbability.
The various bands of chlorophyll in their descending order are chlorophyll (3 chlorophyll a, violaxanthine, xanthophylls and carotene. This proves that chlorophyll is made up of two kinds of pigments, chlorophyll (3 and chlorophyll a.
Common Adsorbants:
Sucrose, cellulose, calcium carbonate, magnesium carbonate, silica and charcoal etc.
Common Solvents:
Petroleum ether, carbon tetrachloride, trichloroethane, benzene, ether etc.
Column:
Column is made up of a glass tube fitted vertically. A slow state of flow is preffered over the fast rate.
Applications of Adsorption Chromatography:
It is extensively used in separation of amino acids, monosachharides, disaccharides, lipids, carotenoids etc.
Electrophoresis:
Electrophoresis was invented by Tiselius in 1937. It is based on the principle that the proteins in a gel migrate in an electric field till they reach their isoelectric point. Isoelectric point is the pH of the medium at which their net charge is zero.
Electrophoresis through a gel separates DNA, RNA and protein molecules. It can be one- dimensional for proteins and nucleic acid separation and two-dimensional for large proteins. Separation occurs on the basis of electrical charge on the molecules when electrodes are provided in the column and electric current is passed through the gel.
Each particle moves towards the electrodes of opposite electric polarity. Movement of the molecules through the gel occurs on the basis of their charge: mass ratio. Small molecules and molecules with greater net charge move faster towards the electrodes in an electric field. Their velocity is directly proportional to the magnitude of the charge on the particle and inversely proportional to the size or mass or molecular weight of the particle.
It is the most common technique for determination of molecular weight of the proteins. Negatively charged molecules migrate across the gel towards the positively charged electrodes and vice versa.
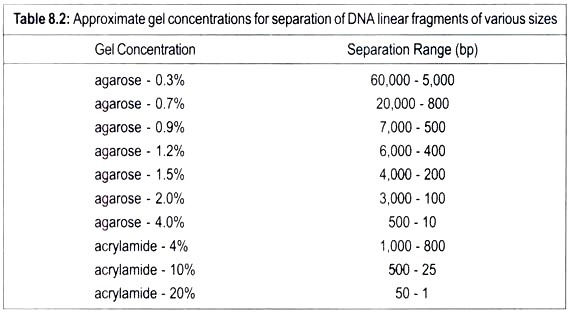
Linear DNA molecules can be separated according to their size in the porous gel matrix when subjected to electric field. As DNA is negatively charged, it migrates towards the positive pole. Smaller molecules travel faster through the gel than the larger ones. Therefor; fragments of different sizes of DNA can be separated because they have moved different distances through the gel.
After completion of electrophoresis DNA molecules are stained with a fluorescent dye ethidium in the gel. Ethedium slips between base pairs. It fluoresces when exposed to ultraviolet light and this enables to visualize DNA. Each band reveals the presence of a population of DNA molecules of a specific size. Two gels polyacrylamide and agarose are used. Polyacrylamide gel electrophoresis (PAGE) is preferred. Linear DNA molecules travel faster than the circular molecules.
Electrophoresis can also separate RNAs. RNA molecules like DNA have also negative charge and often they have secondary and tertiary structures which affect their mobility.
Separation and Purification of Proteins:
Purification of proteins is critical in trending the function of specific proteins. Proteins dissolved in the gel can be easily separated by electrophoresis. Some proteins have greater number of acidic amino acids while there have more basic amino acids in their structure, hence different electric charge on them. Thus difference in their net electric charge enables to separate them.
In two-dimensional electrophoresis, proteins are separated in two steps. In the first step proteins are separated by their net electric charge and later by their masses.
The mixture of proteins is degraded into their individual polypeptides by using urea. This mixture is applied on a gel strip which contains a continuous pH gradient. It is most acidic on one end and most basic on the other end. When an electric current is applied, a charged protein starts moving on the gel strip till it reaches its isoelectric point and becomes stationary. This isoelectric point is the pH at which the net charge on the particle is zero. This technique is called isoelectric focusing as all molecules of a given isoelectric pH migrate to the same region.
In the second step mass of a protein forms the basis for their separation. In the second step, the above strip is placed lengthwise on a polyacrylamide gel slab which is saturated with a detergent, sodium dodecyl sulphate (SDS). It denatures protein molecules changing them from globular compact structure to long flexible polymers. Polymers move in the electric field through the gel with a velocity which is determined by the length of polymers and therefore by molecular weight.
When an electric current is applied to this setup, the proteins migrate from gel strip into the lower gel slab and then separate according to mass of individual proteins. By this technique a large number of proteins can be separated simultaneously. Sodium dodecyl sulphate – Polyacrylamide gel (SDS-PAGE) is a standard procedure for molecular weight determination.