ADVERTISEMENTS:
In this article we will discuss about the Acid-base Balance and Acid-base Imbalance.
Acid-base Balance in Normal Health:
An acid may be defined as substance that produces H+ (protons) in solution, and a base is a particle that combines with hydrogen ions (H+).
Example:
ADVERTISEMENTS:
Carbonic acid is an acid which is dissociated into H+ and HCO3− and its anionic component is a base. NaHCO3 acts as a base since it produces HCO3 which can combine with hydrogen ion (H+).
Other examples:
ADVERTISEMENTS:
Hydrochloric acid (HCl) is a strong acid since it is extensively dissociated into H+ and CI−. Chloride ion (CI−) is a very weak base since it has got very little capacity in combining firmly with H+. But HCO3–, HPO4−−, and protein are comparatively strong bases since they have got strong affinity for H+ forming weak acids.
The stronger the acid, the weaker is the base. Such pairs are termed as conjugate acid base pairs.
Chloride ion (CI−) is the conjugate base of the acid HCl.
The cations such as Na+, K+, Ca++, Mg++, cannot donate or accept protons. So, they are neither acids nor bases. Such cations are termed as aprotes.
Buffers:
Buffers are the mixtures of weak acids and their salts of strong bases.
Example:
(CH3COOH + CH3COONa).
Principles of buffers:
ADVERTISEMENTS:
HAC + NaAC Na+ + H+ + 2AC–
[where HAC = Acetic acid; NaAC = Sodium acetate]
If alkali (NaOH) is added to this system, it will form salt and no free H+ or OH– will be available.
HAC + NaAC + NaOH → 2NaAC + H2O
ADVERTISEMENTS:
If acid (HCl) is added to this system, it will also form salt and no free H+ or OH− will be available.
HAC + NaAC + HCl → NaCl + 2HAC
In either cases, there is no change in hydrogen ion concentration. The buffer acts almost as if it were “absorbing” the added free hydrogen or hydroxyl ions.
Major sources of acids produced in the body:
ADVERTISEMENTS:
i. Carbonic acid (H2CO3):
This acid is produced in the body during oxidation in the cells. The C-compounds on oxidation produces CO2. About 300 litres of CO2 are produced and eliminated from the body of an adult daily.
ii. Sulphuric acid (H2SO4):
This acid is formed by the oxidation of sulphur-containing amino acids e.g., methionine and cystine or cysteine.
ADVERTISEMENTS:
iii. Phosphoric acid:
The dietary phospho-proteins, nucleoproteins, and phosphatides are metabolized to produce this acid.
iv. Abnormal quantities of pyruvic acid, lactic acid, acetoacetic acid, and β-hydroxy-butyric acid are formed from the oxidation of carbohydrates, fats and proteins.
v. When some medicines like mandelic acid, ammonium chloride are administered in excess, they may increase hydrogen ion concentration of blood.
ADVERTISEMENTS:
Mechanisms of regulation of pH:
The following factors are involved in the regulation of blood pH:
i. Buffer systems in the blood.
ii. Respiratory mechanisms.
iii. Renal mechanisms.
ADVERTISEMENTS:
iv. Dilution factor.
Physiological buffer systems:
E.C. fluids are capable of transporting acids from the cells to the lungs and kidneys without the change in pH depending mainly on the efficient buffer systems in these fluids and in the erythrocytes.
The most important blood buffer systems are as follows:
(a) Plasma buffers:
(b) Buffers of red blood cells:
The buffer systems in the interstitial fluids and lymph are almost the same as in the blood plasma except the proteins which are present in muscle in smaller quantities.
The followings are the most important buffers:
(a) In plasma:
The bicarbonate buffer and plasma proteins.
(b) In erythrocytes:
The bicarbonate buffer and Hb-system.
Role of different buffer systems:
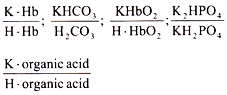
a. Bicarbonate buffer system:
This consists of carbonic acid and sodium bicarbonate. The normal ratio of it in blood is 20 : 1. These chief buffers constitute the alkali reserve. The bicarbonate buffers neutralize the strong and non-volatile acids entering the ECF.
Thus, lactic acid is buffered as follows:
A strong and non-volatile acid (lactic acid) is converted into weak and volatile acid at the expense of NaHCO3 (the salt component of the buffer). H2CO3 thus formed is eliminated through diffusion of CO2 through alveoli of lungs.
Therefore, bicarbonate buffer system is directly linked up with respiration.
When NaOH enters the ECF, it reacts with H2CO3 of the buffer system:
(ii) When an alkali enters, it is buffered by the acid phosphate resulting the alkaline phosphate which is excreted in urine showing the increased alkalinity of urine.
NaHCO3 concentration in the blood is represented as the alkali reserve since it does not combine with strong and non-volatile acid.
Advantage:
Bicarbonate buffer system is quite efficient as compared to other buffer systems since it is present in very high concentration and it produces H2CO3 from which CO2 is exhaled out.
Disadvantage:
Bicarbonate buffer is very weak as a chemical buffer and hence PKa is far away from the physiological pH.
b. Phosphate buffer system:
The normal ratio of Na2HPO4/NaH2PO4 in plasma is 4 : 1 and this ratio is kept constant by the help of the kidneys for which phosphate buffer system is directly related to the kidneys.
(i) When a strong acid enters the blood, it is fixed up by Na2HPO4 which is converted to acid phosphate (NaH2PO4). This acid phosphate is excreted by the kidneys showing that the urine becomes more acidic.
(ii) When an alkali enters, it is buffered by the acid phosphate resulting the alkaline phosphate which is excreted I urine showing the increased alkalinity of urine.
So, phosphate buffer system works in conjugation with the kidneys.
Advantage:
It is very effective as a chemical buffer and PKa approaches physiological pH better.
Disadvantage:
Phosphate buffer is low in concentration in blood and less efficient as a physiological buffer.
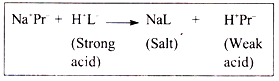
c. Protein buffer system:
The capacity of plasma protein buffer (Na+Pr−/ H+Pr−) is less than that of Hb which is only involved in erythrocytes.
Buffering action of proteins:
(i) In acidic medium, protein acts as a base and NH2 group takes up H+ from the medium forming NH3+. Protein becomes positively charged.
(ii) In alkaline medium, protein acts as an acid and acid group (COOH) dissociates into COO− and H+. This H+ combines with OH– producing a molecule of H2O. Proteins become negatively charged.
(iii) The salt component (Na+ proteinate) combines with strong acid producing weak acid (H+Pr−).
(iv) CO2 is removed by other factor with the formation of carbamino compounds. The binding of CO2 is performed without passing through the carbonic acid stage.
PrNH2 + CO2 ⇌ PrNHCOOH (Carbamino compound)
d. Hemoglobin as a buffering agent:
The capacity of Hb as a buffer fully depends on the number of dissociable buffering groups, namely -COOH group, -NH2 group, guanido group and the important imidazole group.
The physiological buffering action of Hb to maintain the pH range of blood from 7.0 to 7.8 is due to imidazole group of histidine.
Imidazole contains two groups:
(a) Fe++ containing group which is concerned with carriage of O2.
(b) Imidazole N2 group which can give up H+ and accept H+ depending on the pH of the medium.
Therefore, the buffering capacity of Hb entirely depends on the presence of imidazole nitrogen group which remains dissociated in acidic medium and conjugated base forms.
(a) The imidazole N2 group being oxygenated acts as an acid and donates protons in the medium. Oxygenated Hb is a stronger acid than de-oxygenated Hb.
(b) The deoxygenated Hb is less acidic and the imidazole N2 group acts as a base and takes up protons from the medium.
(c) The delivery of O2 is favoured by the acidity of the medium.
(d) The oxygenation of Hb is favoured by the alkalinity of the medium.
In the lungs:
(a) H+ is released during the formation of Oxy-Hb (HbO2) from deoxygenated Hb (H.Hb). This H+ then reacts with HCO3− to form H2CO3.
(b) The low CO2 tension in the lungs shifts the equilibrium towards the production of CO2 which is continually eliminated in the expired air.
In the tissues:
(a) The reduced O2 tension and the local acidity compel oxy-Hb (HbO2) to deliver O2 to the cell forming deoxygenated Hb (H.Hb).
(b) The metabolic CO2 in the cells is hydrated to form H2CO3 which is dissociated to form H+ and HC03–.
The deoxygenated Hb (H.Hb) is formed from oxygenated Hb(HbO2) by the effect of H+ with the little change in pH.
Iso-hydric transport of CO2:
The newly formed H+ in the tissues from H2CO3 does not bring about a change in pH. This circumstance in the role of Hb buffers is sometimes referred to as iso-hydric transport of CO2.
Role of Respiration in Acid-base Regulation:
The respiratory mechanism depends on:
(a) The response of the respiratory centre (RC) in medulla oblongata to a very slight change in pH and pCO2.
(b) The diffusibility of CO2 from the blood into the alveolar air across the pulmonary alveolar membrane.
Therefore, the lungs become healthy for which diffusion of CO2 occurs properly.
(i) 0.2 per cent increase in CO2 results in 100 per cent increase in pulmonary ventilation which causes slight increase in H+ concentration of the blood (acidosis). The excess CO2 is promptly removed from the ECF in the expired air.
(ii) A decrease in H+ concentration (alkalosis) causes depression of respiratory centre with slow and shallow respiration (hypoventilation) resulting in the retention of CO2 in the blood until the normal pCO2 and pH are restored.
Hence, the respiratory mechanism tends to maintain the normal BHCO3/H2CO3 ratio in the EC fluids.
Renal Mechanisms for Regulation of Acid- base Balance:
Kidneys are also involved in acid-base equilibrium.
(a) By eliminating the non-volatile acids, e.g., lactic acid, sulphuric acid, acetoacetic acid, β-hydroxy butyric acid, etc. after being buffered with Na+ through the glomerular filtration.
(b) Na+ exists as NaHCO3 (alkali reserve) in the renal tubules by reabsorption by the renal tubules in exchange of H+ which are secreted.
Three mechanisms are involved in the above mechanism:
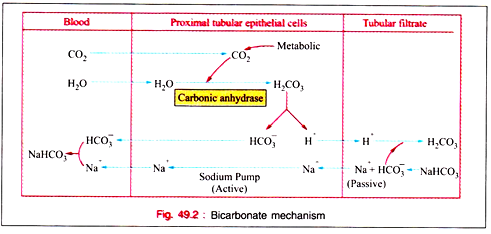
i. Bicarbonate mechanism.
ii. Phosphate mechanism.
iii. Ammonia mechanism.
i. Bi-carbonate mechanism:
(a) Carbonic acid (H2CO3) is ionized as H+ and HCO3− and this H+ is mobilized for tubular secretion. The formation of H2CO3 from CO2 and H2O is catalysed by the zinc- containing enzyme carbonic anhydrase, present in real tubular epithelial cells. Carbonic anhydrase is also present in RB cells, parietal cells of stomach, most of tissues, and small quantities in muscle tissue, pancreas, spermatozoa as per recent information.
(b) All of the bicarbonate ions (HCO3−) are normally reabsorbed by the proximal tubular epithelial cells but a slight excess of hydrogen ions (H+) remains in the tubules to react with other substances to be excreted in urine.
(c) In the tubular filtrate, hydrogen ions are exchanged against sodium ions.
(d) Increase in pCO2 accelerates formation of H2CO3 and thus increases H+ secretion by the renal tubular epithelial cells which help in the reabsorption of HCO3−.
(e) In the excess administration of K+, K+ quickly enters into the cell in exchange of H+ and hydrogen ions are buffered by bicarbonate of ECF for which plasma bicarbonate content is diminished. As a result, the renal tubular epithelial cells secrete less hydrogen ions (H+), and less bicarbonate ions (HCO3−) are absorbed. The excretion of bicarbonate in urine becomes more and the urine becomes alkaline although ECF is acidic.
(f) The clinical importance shows that potassium ions (K+) leave the cell in K+ deficiency and hydrogen ions (H+) enter the cells producing intracellular acidosis. ECF becomes alkaline. Increased excretion of H+, NaH2PO4, and NH4Cl increase the titratable acidity. ECF is though alkaline, highly acidic urine is excreted. This condition is referred to as paradoxic aciduria. This occurs in patients treated for long with cortisone, Cushing’s syndrome, and in patients with potassium free fluids.
(g) Increase in Cr causes decrease in HCO3−.
(h) The enzyme carbonic anhydrase is inhibited by Diamox. The urine becomes alkaline when the drug is administered and increased amount of NaHCO3 appears in urine. There is reduction in titratable acidity and ammonia excretion in the urine. Drug is used clinically as a diuretic to induce a loss of Na+ and water in congestive cardiac failure and in hypertensive heart diseases.
ii. Phosphate mechanism:
(a) The ratio of plasma Na2HPO4 and NaH2PO4 determines the pH of the urine. The ratio of these two in plasma is maintained to 4 : 1 if the concentration of Na2HPO4 exceeds that of NaH2PO4. But the ratio becomes 9: 1 if the concentration of NaHPO4 in urine exceeds that of Na2HPO4.
(b) The pH 7.4 of glomerular filtrate is changed to pH 6.0 or even as low as 4.8 in urine.
(c) Na+ is exchanged for secreted H+ which changes Na2HPO4 to NaH2PO4 resulting the increase in acidity of urine.
(d) This mechanism takes place in the distal tubule of kidney.
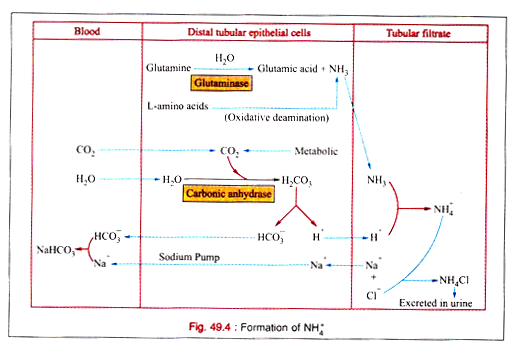
iii. Ammonia mechanism:
Na+ is conserved by the elimination of H+ for the production of NH3 by the renal distal tubular epithelial cells.
Sources of NH3:
(a) Glutamine is hydrolysed to produce NH3 by the enzyme glutaminase present in these cells.
(b) It can be formed from other amino acid by oxidative deamination.
(c) It is also formed from glycine by glycine oxidase
Formation of NH4:
NH3 is diffused to the tubular filtrate where it combines with H+ to form NH4+.
The renal glutaminase activity is enhanced by acidosis. NH3 production is highly increased in metabolic acidosis and negligible in alkalosis.
Acid-base Imbalance in Normal Health:
Acidosis:
A. Metabolic acidosis;
B. Respiratory acidosis.
A. Metabolic acidosis:
(a) Metabolic acidosis occurs when a reduction in plasma HCO3− (BHCO3) takes place. In case, there is the deficit HCO3, the ratio of [HCO33−]/[H2CO3] = 20/1 is decreased, i.e., pH is decreased causing metabolic acidosis.
(b) Acidosis stimulates the respiratory centre causing deep and rapid breathing. This increased ventilation results in CO2 loss and reduction in [H2CO3]. Increased ventilation causes reduction in PCO2 which in turn depresses the respiratory centre.
(c) Renal mechanisms can correct the disturbances by conserving cations and by increasing NH3 formation, H+ excretion in distal tubules, and reabsorption.
Causes:
(i) Abnormal increase in “anions” other than HCO3− caused from:
(a) Endogenous excessive production of acid ions occur in diabetic acidosis, lactic acidosis, starvation, high fever, violent exercise, hemorrhage, shock, and anoxia.
(b) Ingestion of excessive quantities of acids like phosphoric acid, hydrochloric acid, salicylic acid, and mandelic acid, etc.
(c) Renal insufficiency causing retention of acids commonly observed in the terminal stages of nephritis, and destructive renal lesions such as pyelonephritis, pyonephrosis, renal T.B., etc.
The main toots of these causes are:
(a) Decreased H+ – Na exchange.
(b) Decreased NH3 formation.
(c) Decreased glomerular filtration with retention of acid radicals.
(d) Nephritic acidosis due to accumulation of certain organic acids.
(ii) Abnormal loss of HCO3− due to loss of base occurring in the loss of excessive intestinal secretions as in severe diarrheas, small bowel fistulas, and severe biliary fistulas.
B. Respiratory acidosis:
This is caused due to the increase in H2CO3 in the blood following elimination of CO2(PCO2) in the pulmonary alveoli. This results from breathing air containing abnormally high percentage of CO2, and conditions in which elimination of CO2 through lungs is retarded.
Mechanism:
(a) In the depression of respiratory centre, the excretion of CO2 through lungs is impaired causing more accumulation of CO2 in blood for which there is excess formation of H2CO3. So the ratio of [HCO3−]/ [H2CO3] is lowered resulting the decrease in pH.
(b) The increased CO2 tension (PCO2) causes the elevated stimulation to respiratory centre resulting increased ventilation. This mechanism is less effective.
(c) In renal mechanism, more HCO3− are reabsorbed from tubules responding to raised PCO2 in blood and the ratio of [HCO3−]/[H2CO3] is restored towards 20 : 1. This mechanism is an important one.
Causes:
A. Conditions Causing depression of respiration:
(a) Damage to CNS:
(i) Brain damage—trauma, inflammation, and convulsive disorders.
(ii) Drug Poisoning—Morphine and barbiturates.
(iii) Excessive anesthesia.
(iv) Bulbar Polio.
(b) Loss of ventilatory functions:
(i) Tension.
(ii) Pulmonary and mediastinal tumors.
(iii) Emphysema.
(c) Effect of pain, e.g., pleurisy.
B. Conditions causing impairment of diffusion of CO2 across alveolar membrane:
(a) Emphysema.
(b) Pulmonary edema and congenital alveolar dysplasia.
C. Conditions in which there is an obstacle to the escape of CO2 from:
The alveoli:
(a) Obstruction to respiratory tract-Laryngeal obstruction and asthma.
(b) Rebreathing from a closed space.
D. Conditions in which pulmonary blood flow is insufficient:
(a) Certain congenital heart diseases.
(b) Ayerza’s disease.
Alkalosis:
A. Metabolic Alkalosis:
(a) The accumulation of excess of HCO3– causes an increase in the ratio of [HCO3–]/ [H2CO3], i.e., pH is elevated.
(b) Alkalosis inhibits the respiratory centre (RC) developing irregular breathing. This reduced ventilation causes CO2 retention and carbonic acid level [H2CO3] elevation.
(c) Since the levels of both in blood are increased the ratio of [HCO3–]/[H2CO3] are restored towards 20:1.
(d) Decreased ventilation raises PCO2 which stimulates the respiratory centre.
(e) In renal mechanisms, there is the increased excretion of cations, bicarbonates, K+ but reduced excretion of NH3.
(f) There is the decreased urinary acidity and titratable acidity.
Causes:
(a) The excessive loss of HCl from stomach is associated with high intestinal obstruction, pyloric obstruction, pylorospasm in infants.
(b) Na+ and K+ are mainly retained in the body in the form of bicarbonate due to the loss of Cl– from the blood. Thus, a neutral salt (NaCl) is replaced by an alkaline salt (NaHCO3).
(c) Excessive intake of bases like NaHCO3, and acetates, lactates, citrates of sodium and potassium. Lactates and citrates are converted into HCO3–.
(d) Potassium deficiency.
(e) Deep X-ray therapy, radium therapy, and prolonged exposure to ultra violet rays cause a decrease in the H+ concentration in the blood plasma.
(f) Excessive vomiting.
Clinical manifestations:
Alkalosis is observed in:
(a) Decreased ionic calcium in blood producing tetany.
(b) Hypokalemia due to the increased excretion of K+ from the distal tubules.
(c) Kidney damage caused by the degenerative changes in the tubules (nephrosis) occurring with oliguria and nitrogen retention.
(d) Ketosis and ketonuria due to excessive vomiting for the insufficient carbohydrate intake.
B. Respiratory Alkalosis:
Respiratory alkalosis occurs when there is a decrease in [H2CO3] with no change in HCO3 in plasma. As a result, pH is increased.
ADVERTISEMENTS:
Mechanism:
(a) [H2CO3] is reduced as a result of increased loss of CO2 and the ratio of [HCO3–]/ [H2CO3] is elevated for which pH is increased.
(b) Owing to increased loss of CO2 less bicarbonate is reabsorbed by the renal tubules for which the ratio of [HCO3–]/[H2CO3] returns towards normal (20: 1).
(c) The respiratory centre is depressed by alkalosis and PCO2 and CO2 excretion is reduced.
(d) In renal mechanism, there are excretion of alkali in the form of bicarbonate, decreased excretion of acid, decreased excretion of ammonia, and retention of chloride ion (Cr) in the blood.
(e) Other features are the same as that of metabolic alkalosis.
Causes:
(a) The respiratory centre (RC) is stimulated by CNS diseases like meningitis, encephalitis due to hyperventilation in some cases of these diseases manifesting hyperpnea over long periods.
(b) Large doses of salicylate cause stimulation of respiratory centre (RC) with hyperventilation showing alkalosis.
(c) Hyperventilation is caused by the increased respiratory rate associated with increased body temperature.
(d) Hysterical attacks cause hyperventilation and hyperventilation tetany with alkalosis is also observed in hyper-excitable donors.
(e) Hyperpnoea occurring in untrained individuals ascending to high altitudes results in H2CO3 deficit and alkalosis.
(f) Hepatic coma is also another cause of respiratory alkalosis.
(g) Injudicious use of respirator.