ADVERTISEMENTS:
The following points highlight the eight important types of laboratory diagnosis of bacterial diseases. The types are: 1. LEPROSY 2. Brucellosis 3. Streptococcal Sore Throat 4. Infections of Middle Ear and Sinuses 5. Infections of the Lungs 6. Keratitis 7. Pyrexia of Unknown Origin 8. Septicaemia.
Bacterial Disease: Type # 1. LEPROSY:
Mycobacterium leprae, the causative agent of leprosy (also called Hansen’s disease) was first described by Hansen in 1874 in Norway.
Laboratory diagnosis:
ADVERTISEMENTS:
Diagnosis of leprosy is based primarily on clinical signs and symptoms.
Specimens: Skin biopsy and scrapings from lesion and nasal mucosa.
Direct evidences:
I. Direct slit-skin smear and acid-fast staining:
ADVERTISEMENTS:
Slit-skin smears made from skin scraping from patches and ear lobes and punctured material from nodules and nasal mucosal scrapings are stained by modified Ziehl-Neelsen method using 4 to 5% sulphuric acid as decolorizing agent.
Smear shows acid-fast bacilli arranged in parallel bundles within macrophage (Lepra-cell). The living bacilli stain uniformly while the dead bacilli look fragmented and irregular or granular in Ziehl-Neelsen stained smear.
Bacteriological index:
It is defined as number of viable bacilli in a lesion which is assessed from stained smear by oil-immersion lens (x 100) of microscope.
Calculation of bacterial index:
Internationally agreed bacteriological index ranges from 1 to 6+ as shown:
1-10 bacilli in 100 fields = 1+
1-10 bacilli in 10 fields = 2+
1-10 bacilli per field = 3+
ADVERTISEMENTS:
10-100 bacilli per field = 4+
100-1,000 bacilli per field = 5+
More than 1,000 bacilli, clumps and groups in every field = 6+.
Morphological index:
ADVERTISEMENTS:
It is the percentage of uniformly stained bacilli out of the total bacteria present in tissue. It is useful in assessing the prognosis and response to treatment.
II. Skin and nerve biopsy:
Skin biopsy is collected from active edge of the patches and nerve biopsy from the thickened nerve for histological confirmation of tuberculoid leprosy when acid-fast bacilli cannot be demonstrated in direct smear.
Microscopically:
ADVERTISEMENTS:
(a) Tuberculoid leprosy shows infiltration of lymphocytes around the centre of epithelial cells; presence of Langhan’s giant cells; few or no acid-fast bacilli.
(b) Lepromatous leprosy shows predominantly foamy macrophages (lepra cell) with few lymphocytes and no giant cell.
Indirect evidences:
(i) Lepromin test:
ADVERTISEMENTS:
The test is helpful in assessment of prognosis of the disease.
(ii) Serological test:
Serodiagnosis of leprosy involves detection of anti-phenolic glycolipid 1 (anti PGL-1) antibodies. Various serological tests like latex agglutination, Mycobacterium leprae Particle Agglutination (MLPA) test (Grade et. al, 1993) and ELISA have been described.
Bacterial Disease: Type # 2. Brucellosis:
Classification:
A. Major Species:
(1) B. melitensis — typically infects goats and sheep, commonly found in Malta and other Mediterranean countries. It is the causative agent on Malta fever.
ADVERTISEMENTS:
(2) B. abortus — typically infects cattle. It is the most important cause of human brucellosis (undulant fever) in U. K.
(3) B. suis — usually infects pigs and is the most important cause of human brucellosis in swine- rearing areas of USA, Latin America and Denmark.
B. Additional species:
(1) B. canis — infects dogs in USA and humans are occasionally infected.
(2) B. ovis — is responsible for testicular infection of rams in Australia.
(3) B. neotomae — infects desert rodents in USA.
ADVERTISEMENTS:
Laboratory diagnosis:
Specimen:
Blood culture in acute stage of the disease is the most definite method of diagnosis. Urine, sputum and breast milk may yield positive culture as the organisms are intermittently excreted in these specimens. Biopsied lymph node or bone marrow aspirate culture is done in chronic cases. Serology is useful in diagnosing acute infection. Multiple samples of blood should be collected for culture and serological testing.
Culture and isolation:
(1) Blood cultures: Because of the systemic nature of infection, blood cultures are first tried.
(2) When the disease has reached an advance stage, bone marrow aspirate or liver biopsy may be necessary to obtain adequate samples of culture.
Since the organisms may be scanty, at least 10 cc blood (or biopsied material) is cultured in a bottle of liver infusion or glucose serum broth of Trypticase Soy Broth and incubated at 37°C under 5-10% CO2. B. abortus, but not other species, must be incubated under 5-10% CO2 environment.
Sub-cultures are made on liver infusion agar or Trypticase Soy agar every 3-5 days. As growth may often be delayed, culture is retained for six weeks. Brucellae grow faster when the blood culture is transferred to agar medium (e.g. biphasic cultures as in Castaneda’s method).
(1) Castaneda’s method of blood culture provides both liquid (liver infusion broth) and solid media (3% nutrient agar slope) in one bottle. Its use reduces chances of contamination and risk of infection to laboratory personnel.
Since the bottle contains both liquid and solid media, the broth flows over the surface of agar slant when the bottle is tilted. This results in automatic sub-culture. The bottle is kept in the incubator in upright position. In positive cases, colonies develop on the slant. Blood culture comes positive in 30-50% of cases. B. melitensis is more readily cultured than B. abortus.
2. Trypticase Soy broth:
Specimen of blood is inoculated in Trypticase Soy broth and then subcultures on serum dextrose agar every 3-5 days.
3. BACTEC is a rapid and automated diagnostic system for culture in which results are obtained in 5-6 days.
Identification:
Isolated organism is identified by:
1. Requirement of CO2 for growth.
2. Production of urease and H2S.
3. Sensitivity to the dyes basic fuchsin, thionin.
4. Agglutination with mono-specific sera, and
5. lysis by bacteriophage.
Serology:
Because of the difficulty of isolating brucellae, many cases of Brucella infection are diagnosed by serological tests which include: the standard agglutination test (SAT), and an ELISA procedure for detection of Brucella-specific IgM, IgG.
1. Standard agglutination test (SAT):
Diluted serum of patients is tested by slide or tube method against a brucella suspension. This test usually turns positive 7-10 days after infection. Both IgM and IgG antibodies continue to rise during acute stage of infection. A presumptive diagnosis can be made when there is a four-fold rise in the titre or a single titre greater than or equal to 80.
Most patients with active brucellosis show SAT titres of 160 or higher, these titres subsequently fall with adequate therapy. Antigen employed in ST is from B. abortus. Antibodies to B. melitensis or B. suis cross react with B. abortus antigen, however, there is no cross-reactivity with B. canis. Hence, specific B. canis antigen is to be used for diagnosing B. canis infections.
Prozone phenomenon (absence of agglutination in low dilution of serum due to high concentration of antibodies) is common and, hence, a serial dilution of serum is to be tested.
(2) ELISA and RIA:
These tests are valuable and very sensitive and can distinguish IgM, IgG and IgA brucella specific antibodies and are helpful to distinguish acute and chronic brucellosis.
Brucellin test (skin test):
It is an intradermal test done by extract of brucella for detection of delayed hypersensitivity. Positive reaction shows an area of erythema and induration of 6 mm diameter within 24 hours.
Detection of animal infection:
Milk ring test:
It detects brucella agglutinins in the milk of infected dairy cattle and is sufficiently sensitive for testing the bulked milk supply from a herd.
Technique (Fig. 12.8):
1. One ml. column of well-mixed milk is taken in a 3 x 3/8 inch test tube.
2. A drop of concentrated suspension of B. abortus or B. melitensis stained with haematoxylin is added to the column of milk and mixed thoroughly by shaking.
3. The test-tube with milk and bacterial suspension is incubated in a water-bath at 37°C for 40-50 minutes, i.e. sufficient time for the cream to rise.
Result:
In a positive test, the bacteria are agglutinated and rise with the cream to form a blue line above the white skimmed-milk. When brucella agglutinins are absent, the cream line is white and lies above a blue milk column.
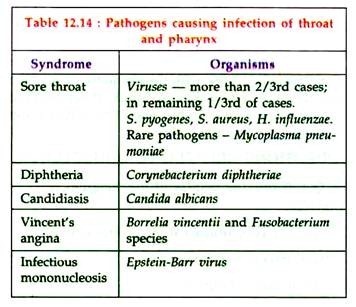
Bacterial Disease: Type # 3. Streptococcal Sore Throat:
Streptococcus pyogenes causes streptococcal sore throat. other organisms causing sore throat are listed in table 12.14:
Laboratory diagnosis:
Specimen:
Throat swab from fauces.
Culture:
Blood agar incubated aerobically and anaerobically.
Identification:
(i) Colonies — small, dry, beta-haemolytic.
(ii) Lancefield grouping.
Latent period:
2-3 but up to 6 weeks after throat infection.
Rheumatogenic serotypes:
Any serotype, M types 5, 18, 24 more frequently incriminated.
Serology:
ASO titre 200 (significant) or more.
RF prone to repeated attacks, hence prophylaxis with penicillin is necessary on a long-term basis and is mandatory to eliminate streptococci.
Laboratory diagnosis of other causes of sore throat — e.g. Diphtheria, Vincent’s infections.
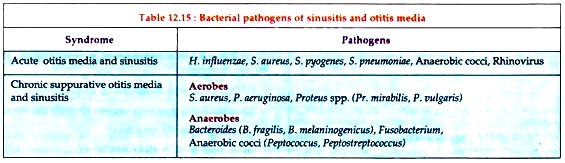
Bacterial Disease: Type # 4. Infections of Middle Ear and Sinuses:
Important causative agents of sinusitis and otitis media are listed in Table 12.15.
Laboratory diagnosis:
Specimen:
Swabs or pus, antral washings (collected carefully for anaerobic culture by disposable sterile syringe, needle of which bent at right angle immediately after collection).
A. Microscopy: Gram-stained film is not helpful.
B. Culture: Blood agar, nutrient agar, RCM. Incubation – aerobically and anaerobically.
C. Other tests: Coagulase test – S. aureus, Oxidase test – Ps. aeruginosa, urease test – Proteus spp. Biochemical tests help to distinguish different species.
Bacterial Disease: Type # 5. Infections of the Lungs:
Causative agents of laryngitis and tracheitis are shown in Table 12.16:
Laboratory diagnosis:
Specimen:
Sputum:
Early morning sample which is most purulent. A properly taken specimen with minimal salivary contamination is to be sent to the laboratory as early as possible. Specimen of blood for culture may be useful in diagnosis of lobar pneumonia.
Microscopy:
1. Gram-film:
Adequate number of pus cells and presence of predominant organisms gives a clue to the probable pathogen.
2. Ziehl-Neelsen or Auramine film:
Presence of acid and alcohol—fast bacilli offers a presumptive diagnosis of tuberculosis.
3. Silver methenamine stain of bronchial lavage and washings for P. carinii.
Culture:
Purulent portion of sputum is best for culture.
If the sputum is too viscid, it may be homogenized by treatment with a liquefying agent that also allows semi-quantitative culture:
1. Blood agar:
It is incubated aerobically at 37°C to look for predominant isolate after assessment of the normal bacterial flora.
2. Chocolate agar:
It is a selective medium for H. influenzae. The inoculated media is incubated at 37°C in air with 5-10% CO2.
3. Blood agar:
Inoculated medium is incubated at 37°C anaerobically with 5-10% CO2.
Culture for mycobacteria:
It is done only where indicated. The material is inoculated on egg containing media in screw-capped containers to preserve moisture over the long incubation period of 6-8 weeks. Three samples of sputum to be collected on successive days for culture on Lowenstein-Jensen medium which are then incubated for 6-8 weeks.
Detection of bacterial antigens:
A rapid diagnosis of Pneumocystis carinii and Legionella pneumophila can be made by fluorescent antibody staining of bronchial lavage and respiratory secretions, respectively.
Serological tests:
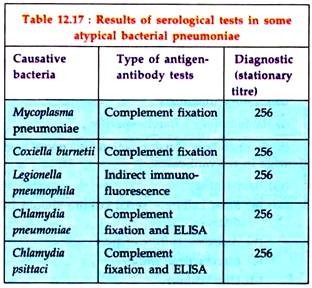
Since the isolation of some causal organisms are difficult to make, all suspected cases should be serologically investigated by demonstration of antibody in the patient’s serum (Table 12.17). Most often, such cases are diagnosed by stationary high titres, although demonstration of a four-fold rising titre of antibody is better.
Bacterial Disease: Type # 6. Keratitis:
Causes:
Mostly herpes virus and foreign body with secondary infection due to bacteria or fungi.
Laboratory diagnosis:
1. Bacterial infections except chlamydiae:
Specimen consists of exudate, collected by a sterile platinum loop directly from the patient’s eye.
Diagnosis:
Microscopy of Gram-stained smear and culture in blood and chocolate agars are useful to detect the organism.
2. Chlamydial infections:
Specimen includes scrapings from conjunctiva.
Diagnosis:
Microscopy of Giemsa staining or immuno-fluorescent staining of smear shows cytoplasmic inclusions. Chlamydia grows in tissue culture (McCoy cells).
3. Mycotic infections:
Specimen consists of scrapings from the base of edge of corneal ulcer.
Microscopy:
In 10% KOH mount and methenamine silver or PAS staining, the fungi appear as branched septate hyphae.
Culture:
Scraping material is inoculated on Sabouraud dextrose agar.
Bacterial Disease: Type # 7. Pyrexia of Unknown Origin (PUO):
Causes:
Sometimes the cause of a febrile illness (temperature greater than 38°C) remains uncertain in spite of investigations and such a case is known as PUO (pyrexia of unknown origin).
In the classic studies of PUO, only patients with persistent fever for 3 weeks or longer were included. According to some, the patient with PUO must have persistent fever for at least one week; usually longer, and often three weeks. The important causes are shown in Table 12.18.
Laboratory diagnosis:
(i) Haematology:
Rarely specific.
(ii) Biochemistry:
Liver function tests reveal liver damage in relevant cases.
(iii) Bacteriology:
(a) Blood culture – serial culture should be done.
(b) Anaerobic culture of pus.
(c) Mycobacterial culture.
(d) Urine culture.
(iv) Serology:
Useful in infectious mononucleosis (Paul-Bunnell test), enteric fever, hepatitis A, B infection, Lyme disease, CMV infections and sometimes in amoebiasis.
(v) Skin tests:
Mantoux test and tests for histoplasmosis, coccidioidomycosis, sarcoidosis (Kveim- Siltzbach skin test).
(vi) Other tests:
(a) Immunologic tests such as LE cell phenomenon, antinuclear antibody test in SLE, etc.
(b) Biopsy of lymph node or other tissues.
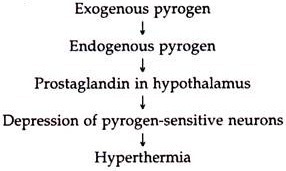
Principle of action:
Bacterial Disease: Type # 8. Septicaemia:
Causative organisms:
Septicaemia as complications of more localised lesions is much more common than the generalized infections.
Organisms most commonly isolated from cases of septicaemia include:
1. Gram-negative bacilli (60-70% cases):
(i) Salmonella:
(a) S. typhi,
(b) S. paratyphi A.
(c) S. paratyphi B.
(d) S. paratyphi C.
(ii) Brucella spp.
(iii) Haemophilus influenzae.
(iv) Escherichia coli.
(v) Klebsiella pneumoniae.
(vi) Proteus mirabilis and P. vulgaris.
(vii) Enterobacter spp.
(viii) Bacteroides spp.
2. Gram-positive cocci (20-40% cases):
(i) Staphylococcus aureus.
(ii) Streptococcus pneumoniae.
(iii) Streptococcus pyogenes.
(iv) Streptococcus faecalis.
3. Gram-positive bacilli:
Laboratory diagnosis:
Blood culture is the main diagnostic procedure. Sample should be collected before initiating antimicrobial therapy.
1. Specimen:
Three to six samples of blood, 10 ml each to be collected from antecubital vein over 24 hours. Sample of blood should be directly inoculated into a large volume (50-100 ml) of medium (glucose broth). One culture should be obtained from each vein puncture, using anaerobic as well as aerobic techniques. Addition of pyridoxal hydrochloride improves the chances of isolating nutritionally deficient variant streptococci.
Utility of larger volume of blood and media:
Following are the advantages:
(i) Number of organisms in the blood may be very few, even as few as one/ml.
(ii) Dilution of bactericidal substances present in blood.
2. Culture:
Processing of culture:
(a) After collection of blood in blood culture bottles these are incubated aerobically and blind subcultures are made after 24 hours, 48 hours, 72 hours, on 6th day and 10th day.
(b) Subcultures are made on:
(1) Blood agar.
(2) MacConkey’s agar.
(3) Chocolate agar.
(4) Anaerobic supplemented blood agar for anaerobes.
(5) Sabouraud’s dextrose agar for fungi.
(6) Other selective media as indicated.
One blood agar is to be incubated anaerobically, chocolate agar under 5-10% CO2 and other media under ordinary laboratory conditions. Colonies obtained on solid media after incubation are studied further for identification by the help of biochemical reactions and antibiotic susceptibility by Kirby- Bauer’s disc diffusion method.
Cultures should be incubated at 37°C and to be observed daily for early signs of growth making periodic (daily or on alternate days) blind Gram- stains and subcultures. Cultures should not be discarded as negative for at least 3 weeks. Subculture is made on blood agar aerobically and anaerobically.
Castaneda’s method of culture of blood is likely to yield better result.
Identification:
The isolated organism is identified by biochemical and agglutination tests.
3. Antibiotic sensitivity test:
Laboratory monitoring of therapy is necessary for which the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the antimicrobial agent for the isolated organism must be determined.
The routine disc diffusion technique with a fixed concentration of drug is not adequate for guiding treatment of infective endocarditis. The measurement of MIC and MBC of antibiotic helps to determine adequate dose of the antibiotic to be used for ensuring the serum levels that can penetrate the valves and kill the organisms.
Culture negative endocarditis:
In about 10-20% cases, blood culture yields persistently negative result, several reasons are suggested for the same:
(i) Recent antibiotic therapy.
(ii) Infection by fastidious or slow-growing organisms, e.g. Haemophilus spp., Bacteroides, Actinobacillus spp. or nutritionally-variant streptococci will not grow on standard medium.
(iii) Bacteraemia is intermittent.
(iv) Infection with Coxiella burnetii or Chlamydia psittaci: Blood cultures may be negative but the diagnosis is established by serological tests (CFT) for both Phase I and Phase II antigens. Titres are usually above 250 and even greater.
4. Other tests:
(i) Echocardiograms demonstrate the vegetation’s and valve changes in 75-80% cases of native valve endocarditis.
(ii) Leucocytosis and elevated ESR are common but not invariable. Measurement of C-reactive protein in blood is sometimes more reliable than ESR in making assessment of progress.
Impaired Host Defenses:
(a) Deficiency of cell-mediated immunity.
(b) Deficiency of humoral immunity.
(c) Decreased complement level.
(d) Diminished phagocytic activity.
(e) Poor natural killer cell activity.
(f) Diminished inflammatory response.
(g) Malignancy,
(h) Diabetes mellitus.
(i) Congenital inflammatory response.
(j) Severe neutropenia
(k) Intravenous drug abuse.
(l) Prematurity and low birth weight,
(m) Old age.
(n) Splenectomised patients.
(o) Immunosuppressive therapy.
(p) Uraemia,
(q) Aplastic anaemia.
(r) Hepatic failure.
ADVERTISEMENTS:
(s) Nephrotic syndrome,
(t) Myeloma.
Pre-existing infections:
(a) Abdominal sepsis—peritonitis, liver abscess.
(b) Pneumonia due to Gram-negative bacteria.
(c) Gram-negative infections of burns, wounds, abscesses, etc.
(d) Meningitis.
(e) Gynaecological sepsis—puerperal sepsis, pelvic abscesses, salpingitis.
(f) Food poisoning.
(g) Osteomyelitis, septic arthritis, etc.
(h) Urinary tract infections.
Instrumentation and surgery examples:
After surgery on urinary tract, gastrointestinal tract, etc. radiological invasive techniques—catheterisation, cystoscopy, prosthetic devices, endotracheal tubes, etc.
Other factors:
Like difficult delivery, exposure to infected incubators, exposure to infected ventilators, etc.