ADVERTISEMENTS:
In this article we will discuss about:- 1. Definition of Growth 2. Measurement of Bacterial Growth 3. Multiplication of Unicellular Bacteria 4. Determination of Generation Time 5. Growth Curve 6. Continuous Culture 7. Synchronous Culture 8. Culture Media 9. Enrichment Culture 10. Requirements of Macro- and Micro-Elements for Growth 11. Physical Factors Influencing Growth.
Contents:
- Definition of Growth
- Measurement of Bacterial Growth
- Multiplication of Unicellular Bacteria
- Determination of Generation Time
- Growth Curve
- Continuous Culture
- Synchronous Culture
- Culture Media
- Enrichment Culture
- Requirements of Macro- and Micro-Elements for Growth
- Physical Factors Influencing Growth
1. Definition of Growth:
In biology, growth is generally defined as an irreversible increase in cellular mass due to active synthesis of all the constituents. Growth results in increase of cell number (except in coenocytic organisms). In multicellular organisms, this increase in cell number leads to increase in size, because the daughter cells remain together.
ADVERTISEMENTS:
In contrast, in unicellular organisms, cell multiplication leads to increase in number of individuals in a population, i.e. the population size. So, for bacteria, majority of them being unicellular organisms, growth may be defined as the increase in number of cells in a population. It should be remembered, however, that in bacteria and other unicellular organisms, too, a young daughter cell grows in size before it attains a stage when it can divide to complete a cell-cycle.
2. Measurement of Bacterial Growth:
Since bacterial growth results in increase in number and, therefore, in population size, several alternatives are available for its measurement. The cell density i.e. number of cells per unit volume of medium may be determined by counting, by measuring the optical density, by estimating the dry weight or protein, etc. Some of these are direct methods and some are indirect.
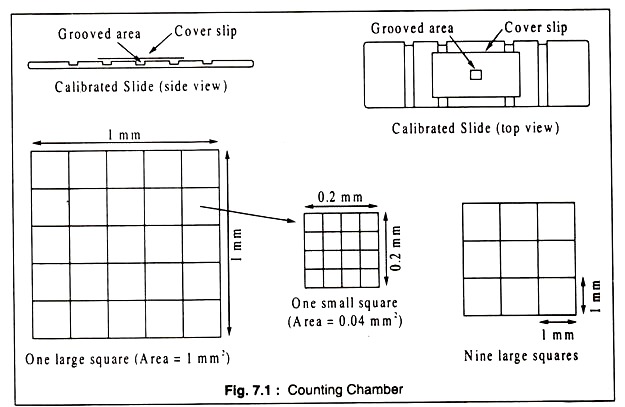
One of the obvious ways for measurement of growth of a growing population in culture (cultivation of organisms in the laboratory on media in which they can grow) is their direct counting under the microscope. The number of cells in a given volume of the culture can be counted using specially ruled grooved slides similar to those used by medical technicians for counting blood cells.
The grooved portion of the slide has a known depth and area which is divided into several equal squares. These slides are called counting chambers, different types of which are available, like Petroff-Hausser, Neubauer, Thoma etc. (Fig. 7.1).
A drop of the bacterial suspension is placed on the groove of the slide, covered with a cover slip, lightly pressed to remove excess fluid and the slide is examined under the high-power objective of a phase-contrast microscope. The number of bacteria per small square is counted for fairly good number of squares, averaged, and from the mean the total number of bacteria per ml of the suspension is calculated.
In Petroff-Hausser counting chamber an area of 1 mm2 is divided into 25 equal squares and the depth of the groove is 0.02 mm. If the number of bacteria is too high for counting, the original culture may be suitably diluted and for calculating the final count the dilution factor is taken into account. Motile bacteria are to be immobilized before transferring to the counting chamber.
The number of bacteria obtained by the above procedure gives the total count. It is obvious that the total count includes both living as well as dead cells. Living or viable bacteria are capable of producing progeny cells by division.
On a solidified nutrient medium a viable bacterium divides and re-divides to form a large number of progeny cells to form a colony. So, the colony-forming ability is a test for viability. A dead or non-viable bacterium is unable to form a colony under any condition of growth. The number of living bacteria per unit volume of a culture or a population is known as its viable count.
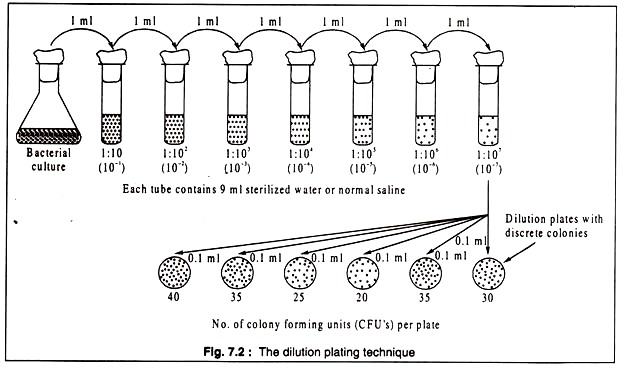
The method followed for determining viable count is based on the principle of serial dilution, first developed by Joseph Lister and later perfected by Robert Koch. There are two variations of the dilution plating technique — the spread plate and the pour-plate methods. In the first method, a measured quantity of a serially diluted sample of the original culture is spread evenly on the surface of the solidified growth medium.
On incubation at an optimum temperature, each viable bacterial cell forms a discrete colony on the surface of the medium. Assuming that each visible colony is the progeny of a single bacterium, the total number of colonies is taken as the number of viable cells present in the quantity of the diluted sample spread on the medium. So, by counting the number of colonies, the viable count can be easily calculated. For reliable results, a good number of replications are necessary.
The method is diagrammatically shown in Fig. 7.2:
[One ml of the bacterial culture containing ~ 107— 109 cells/ml is transferred aseptically with a sterilized pipette to 9 ml of sterilized water or normal saline, mixed thoroughly to get a uniform suspension. Then 1 ml of the 1:10 dilution of the original culture is transferred with a fresh sterile pipette to another tube containing 9 ml of water or normal saline to obtain 1:100 (102) dilution.
ADVERTISEMENTS:
The process is continued till a dilution of 10-7 is reached. Next 0.1 ml aliquots of the final or last two dilutions are uniformly spread with a sterile bent glass rod over the surface of petridishes containing suitable agar medium. On incubation, colonies of bacteria appear on the plates. Number of colonies on replicate plates is counted, their mean determined and the viable count is calculated, taking into consideration the dilution factor. In the example shown in the figure, the viable count comes to 30.7 x 108 = 3.07 x 109/ml.]
For making counting of colonies easier, an instrument called a colony-counter may be used. Essentially, it is a box with a slanting top containing a circular hole of 10-12 cm diameter covered with a frosted glass. There is arrangement for illumination inside the box.
For facilitating counting of small colonies, a magnifying glass of about the same diameter as that of the hole is clamped on a bracket. For counting the petridish is placed in an inverted position on the frosted glass, illuminated from below and observed through the magnifying glass. A marker pen may be used to record the colonies while counting, so that the same colony is not counted more than once.
The second variation of the serial dilution method, the pour-plate technique, is essentially similar to the dilution plating technique, except that the diluted cell suspension is mixed with molten agar medium just before pouring in plates. The temperature should not be so high as to kill bacteria and not so low that agar begins to solidify.
ADVERTISEMENTS:
Good quality agar sets at a temperature of about 42°C, so 45°-50°C is ideal for pouring. On incubation, colonies appear first on the surface. Gradually, bacteria caught inside the agar also form colonies. These colonies are at first lens-shaped before they emerge on the surface. The number of colonies can be counted as in case of spread plates.
The above two methods can be applied for counting aerobic bacteria only, but not for anaerobic bacteria which are unable to grow under normal oxygen concentration of air. For such organisms the dilution plates have to be incubated under oxygen-free atmosphere.
Common vacuum desiccators in which air is replaced by an inert gas like nitrogen can be used. For highly oxygen-sensitive anaerobes, an oxygen-absorbent like alkaline pyrogallol may have to be additionally used. Also, various types of anaerobic jars are commercially available for this purpose.
A different method for determining viable count is the membrane-filter technique. It is mostly used in case of naturally occurring microbial populations, where the cell density is not high (generally -106 cells/ml), such as for counting coliform bacteria in water samples.
ADVERTISEMENTS:
A measured volume of the sample is passed through a sterile membrane filter under negative pressure. The filter should have a pore size less than the diameter of average bacterial cells (less than 1μm). After filtration, the membrane filter disc is aseptically transferred on the surface of suitable sterilized agar medium and incubated till visible colonies appear on the filter surface. The colonies are then counted and the viable count per unit volume of the sample is calculated in the usual way.
An entirely different procedure based on statistical principle is the determination of the most probable number (MPN) of a specific group of microorganisms in natural population. The procedure is generally applied in bacteriological analysis of water samples, but it may be also used for other groups of naturally occurring organisms.
For determination of the MPN of coliform bacteria in a sample of water, aliquots of 10 ml, 1 ml and 0.1 ml of the water sample are inoculated into 5 replicate tubes of lactose broth, each containing an inverted Durham’s tube.
Coliform bacteria ferment lactose to produce gas which collects in the Durham’s tubes.
ADVERTISEMENTS:
After incubation for 48 h at 32°C, the tubes are examined for presence of gas, and the number of tubes showing gas accumulation is counted (Fig. 7.3):
The most probable number of coliform bacteria in the water sample is then determined from a standard MPN table (Table 7.1):
While counting bacteria in a population is one way for determination of growth, another parameter — viz. measurement of bacterial mass — can also be utilized. Gravimetric estimation of fresh bacterial cells or of dry cells provides a direct method.
Fresh weight of a bacterial mass can be obtained by collecting the cells through centrifugation, washing them with distilled water to remove the soluble ingredients of the culture medium and transferring the pellet to a pre-weighed container. For determination of dry weight, the washed cell mass is kept at 110°C overnight and weighed. Very careful handling is essential for obtaining accurate result, since the quantity is very small for laboratory-scale cultures.
ADVERTISEMENTS:
Other methods are also available for measurement of bacterial mass, though they are somewhat indirect. One of these is estimation of total nitrogen of the cell mass by the micro-kjeldahl method. Total nitrogen content of an actively growing culture increases linearly with cell mass.
A similar relationship also exists between total carbon and cell weight. Total carbon content can be estimated by the Van Slyke-Folch method. For routine purpose, estimation of total protein of an aliquot of the culture by any of the common methods gives satisfactory and reliable results.
For total protein, the cells are lysed by treatment with alkali resulting in release of the cell contents, followed by centrifugation to eliminate the cell debris and treating an aliquot with a colouring reagent, like biuret, Folin, Coomassie blue etc.
The colour developed is then measured in a colorimeter. From the colorimeter reading, the quantity of protein (mg/ml) is then read off from a standard curve. A standard curve is prepared using an authentic protein like bovine serum albumin and the same coloring reagent. A standard curve shows known quantities of the protein in one axis and the corresponding colorimetric readings in the other axis.
An indirect but rapid and handy method of estimating cell mass is turbidemetry. Cell mass which is directly proportional to the population density can be accurately measured by this method. As the cell number increases with growth, a bacteriological culture medium turns more and more turbid, allowing less and less light to pass through and at the same time scattering more and more of light by the suspended cells.
For most common bacteria, visible turbidity appears when the cell density is between 106 to 101 cells/ml. When the culture contains less cells, turbidimetric measurement of growth is not feasible. The instrument used for this method is called a photo-electric colorimeter which measures the optical density of a suspension or also the colour density of a coloured solution.
ADVERTISEMENTS:
The optical density of a bacterial suspension is measured by transferring a portion of a liquid culture to a colorimetric glass tube usually having a diameter (light-path) of 1 cm against a blank (control) which is usually the uninoculated culture medium. A suitable light filter (usually green or blue) is used. Optical density can be measured either in terms of absorbance (A) or transmission (T%).
Generally, a colorimeter has both types of scales. Absorbance is the logarithm of the ratio of intensities of incident light (I0) and transmitted light (1) i.e. A = log (I0/I). It is given in log scale. Transmission percent is the percentage of incident light passing through the suspension and is calibrated in an arithmetic scale. The two scales run obviously antiparallel e.g. the blank has an absorbance of O and T% of 100.
As turbidity increases absorbance increases and transmission % decreases. The colorimeter is provided with a light source, some filters for selecting light of a desired range of wave lengths, a colorimeter tube with known light-path, a photo-cell which converts incident light to electric current, a device for amplification of the current so produced and finally a galvanometer for measurement of the electric current (Fig. 7.4). A linear relationship between bacterial cell density and absorbance or transmission exists only when the suspension is relatively thin (Fig. 7.5).
Another instrument known as a nephelometer has more or less the same components as a colorimeter, but is more sensitive. It measures the light scattered by the bacterial cells in suspension. The higher the cell density more is the quantity of scattered light.
The apparatus is so designed that it can capture the light rays scattered by the suspended bacteria at right angles to the beam of incident light and convert them into electricity which can then be measured in the usual way.
Both colorimetry and nephelemetry can be used for measurement of growth of only unicellular organisms which form a stable, uniform suspension. If colorimeter reading or nephelometer reading is calibrated against cell number or cell mass, these methods provide reliable means for estimation of growth.
3. Multiplication of Unicellular Bacteria:
Majority of bacteria are unicellular organisms and most of them multiply by binary fission which means that each bacterium divides to produce two identical cells. Each of them, after attaining maturity, undergoes similar binary fission to produce two daughter cells. Thus, under ideal conditions, cell number as well as mass double itself after given time intervals depending on the species and on the growth conditions.
The time interval between two successive divisions is called the generation time or doubling time. After each unit generation time the number of bacteria doubles. Thus, under optimal conditions, growth of bacteria takes place by geometric progression with a constant factor of 2. Starting from a single bacterium, the increase in number can be represented as 20—>21—>22—>23—>24—>25—>….—>2n after n divisions.
4. Determination of Generation Time:
If the number of cells per unit volume of an actively growing bacterial culture at a time to is taken as N0, then the number at time t during which n divisions have taken place is given by the equation:
Nt = N0.2n, where N, represents the number of bacteria at time t.
Expressed logarithmically, the above equation becomes:
It is seen from the last equation that by counting the number of bacteria present in an actively growing culture at time t0 and t, it is possible to determine the number of divisions that has occurred during the time interval between t0 and t.
Once this is done, the generation time (g) – which is the time between two successive divisions – can be easily determined, because g = t/n. Again, the number of divisions per hour which is known as the doubling rate (v), becomes v = n/t and also v = 1/g. An example may be given for clarification of the above mathematical considerations.
If an actively growing culture has a cell count of 104/ml at a certain time and which increases to 108/ml after a lapse of 6 hours, then the organism has a doubling rate (v):
And the generation time (g) is
The generation time varies from one species to another and it also depends on the growth conditions. Under optimal conditions, Escherichia coli has a generation time of 20 min which means a single E. coli cell can produce 1,024 cells after 200 min during which 10 divisional cycles have taken place.
Exponential or Logarithmic Growth:
When, in a bacterial culture, the population doubles at each generation, the growth is said to be exponential or logarithmic, because the population increases as an exponent (power) of 2 and log2 of the number of cells increases in direct proportion to time. A semi-logarithmic plot of the cell number per unit volume of an exponentially growing population against time gives a straight line (Fig. 7.6).
The linear relation between logarithm of cell number and time holds good, only when all the cells in a growing culture are viable. In practice, such an ideal behaviour is hardly expected, because some cells lag behind or become non-viable.
5. Growth Curve:
Bacterial growth in a flask — or any other container which can be as big as an industrial fermenter — holding a growth supporting medium is known as a batch culture. The batch culture represents a “closed” system, because the nutrients initially present are gradually consumed during growth-producing metabolic end-products which accumulate in the culture vessel causing change of pH value. There is no provision for addition of fresh nutrients or for removal of end products or adjustment of the pH value which also changes during growth.
When the logarithm of the number of bacteria per unit volume of such a batch culture is plotted against time (hr) beginning with the transfer of some viable organisms to the culture vessel (inoculation) a sigmoid growth curve is obtained (Fig. 7.7).
A typical growth curve shows several distinct phases. These are known as the lag phase, the exponential or logarithmic phase, the stationary phase and the death phase. Sometimes, the later part of lag phase is called the acceleration phase and the early part of the stationary phase is called deceleration phase.
The lag phase represents the time interval between inoculation and the onset of the exponential growth. During the lag phase, the cell number does not increase, but individual bacteria grow in size due to active synthesis of cellular ingredients like protein, nucleic acids and carbohydrate.
During the later part of this phase, some of the bacteria begin to divide resulting in a slow rise in total count (acceleration phase). There may be several reasons why the bacteria do not start growing immediately after inoculation in a fresh medium.
If the inoculum is taken from an old culture, or from a culture which was growing in a medium having a different composition, the inoculated cells require some time to adapt themselves in the new environment. So, the lag phase may be considered as an adaptation phase during which the inoculated cells do not remain idle, but they are engaged in synthesizing cell materials as a preparation to initiate active growth.
From the lag phase, the bacteria pass into the exponential phase (logarithmic phase) through the intermediate acceleration phase and they are now fully equipped to initiate growth at a maximum rate. Most cells of the population divide regularly at an interval of each unit generation time resulting in exponential increase of cell number and mass.
However, all cells of the total population do not divide simultaneously, rather they divide asynchronously. As a result, the cell number does not increase in a step-wise manner. The maximal growth rate is maintained throughout the exponential phase and it continues till a point is reached where the population density becomes so high that the growth medium is unable to support further growth.
The growth rate falls and ultimately stops. At this point, the culture enters into the stationary phase. Duration of the exponential phase depends not only on availability of nutrients, but also on other factors, like oxygen supply, pH, temperature etc. and, naturally, also on the generation time. The logarithmic (log) phase cells exhibit their highest activity and, therefore, they are most suitable for measurement of generation time, various biochemical properties and cell size.
As the culture enters into the stationary phase, there is no net increase in cell number, although a majority of the bacteria still remains in a viable state and they continue life activities at the cost of reserve substances stored in the cells.
The duration of this phase is highly variable among different species and may be few hours to several days. From practical point of view, the stationary phase cells are of special significance, because many secondary metabolic products, for example antibiotics, are produced in this phase. Also, for organisms which are cultivated as source of biomass, harvesting in this phase gives maximum yield.
The stationary phase is succeeded by the death phase during which the viable cell count falls exponentially, although total cell count may continue unchanged for quite some time. Thereafter, the total count also falls indicating that the cells undergo lysis and they disappear. Lysis may be due to the activity of their own enzymes (autolysis). It is possible that the cell materials released by lysis may provide nutrition to the still living cells for some time. However, the exact causes of death of bacteria are not clearly understood.
Yield:
An important parameter of growth is the yield which can be determined from the growth curve of a batch culture by measuring the dry weight of the total population at the beginning and at the end of the exponential phase. Thus, if Mmax denotes bacterial mass (dry weight, g) at the end of the exponential phase, and M0 the initial mass, then yield M is, M = Mmax – M0 (Fig. 7.8).
Usually, yield is expressed in relation to the substrate consumption as yield coefficient (Y) which is the ratio of yield (dry wt. g) and the quantity (g) of substrate utilized. Conventionally, yield coefficient per mole of substrate consumed is called molar yield coefficient (Ym).
Another form of expression of the yield coefficient is the energy yield coefficient (YATP) which is yield per mole of ATP consumed. For calculating this parameter it is essential to know the pathway of carbohydrate (energy source) breakdown of the particular organism and the quantity (moles) of ATP produced per mole of the carbohydrate.
For example, Streptococcus faecalis or Saccharomyces cerevisae breaks down glucose by the EMP (Embden-Meyerhof-Parnas pathway) and, when growing in absence of air, produces 2 moles of ATP/mole of glucose.
While molar yield coefficient (Ym) for both these organisms is 20, i.e. both produce 20 g dry cells per mole of glucose (180 g), energy yield coefficient (YATP) for both is 10. Under aerobic conditions, the yield increases significantly, because of higher energy production by complete oxidation of the substrate.
6. Continuous Culture:
The fate of a microbial population growing in a “closed” system like a batch culture is comparable to the fate of a multicellular organism having birth, growth, maturity, and death. The environment in a batch culture is subject to constant changes due to continuous depletion of nutrients, shifting of pH value, partial pressure of dissolved gases, accumulation of toxic metabolites etc.
Due to such changes, the duration of active growth phase is also limited. For many critical experiments, it becomes necessary to maintain a culture in an active state of growth over a prolonged time. This can be achieved by a continuous culture which provides for growth of an organism in a constant environment. A continuous culture represents an “open” system in contrast to a batch culture.
One of the devices for growing microorganisms in a continuous culture is a chemostat developed by Novick and Szilard (1950). In its simplest form, a chemostat consists of a culture vessel provided with an inlet for regulated entry of fresh culture medium, an arrangement for pumping in sterilized air, and an Outlet for maintaining a constant volume of liquid in the culture vessel (Fig. 7.9).
The arrangements in a chemostat ensure a constant regulated flow of fresh medium into the culture vessel and simultaneous removal of equal quantity of culture fluid from the vessel through an outlet device. As a result, a constant volume is always maintained in the vessel.
The system is also provided with an arrangement for pumping in sterilized air through the culture to maintain adequate aeration. In contrast to a batch culture, a continuous culture is more amenable to control. On one hand, the continuous inflow of fresh medium-prevents depletion of essential nutrients and, on the other hand, continuous outflow removes spent medium containing toxic metabolites and bacterial cells. These arrangements ensure a prolonged growth in a chemostat.
The importance of a chemostat culture lies in the fact that the growth rate of an organism can be controlled. This can be achieved by supplying one of the essential nutrients, like carbon and energy source, at a sub-optimal concentration in the inflowing medium.
As a result, the organisms present in the culture are starved and are unable to grow at maximum growth rate. At the same time, the inflow rate can be so adjusted that the particular growth-limiting nutrient is immediately and fully consumed by the hungry organisms. The outflow removes continuously a fraction of the population to maintain a constant cell density.
Thus, it becomes possible to establish a steady-state of growth. In a steady-state, the growth rate is constant and it equals the rate of inflow of medium which is known as the dilution rate. The dilution rate is so adjusted that the concentration of the critical nutrient in the chemostat is practically nil, because the nutrient is instantaneously consumed by the starving population.
As a result, the growth rate in the chemostat is maintained at a sub-maximal value. Thus, by regulating the dilution rate, it is practically possible to maintain a steady-state culture almost indefinitely which is in sharp contrast to a batch-culture which passes through different growth phases — ultimately terminating in death.
The relation between substrate concentration and growth rate is closely comparable with the relation between substrate concentration and velocity of enzyme reaction as depicted by the famous Michaelis-Menten equation. The growth rate (μ) increases linearly with substrate (growth limiting nutrient) concentration [s] up to a certain point, and then declines to become flat. The substrate concentration at which the growth rate is half of the maximum rate of growth is designated by Ks (Fig. 7.10).
7. Synchronous Culture:
A synchronous culture is one in which all the cells of a particular population are in the same stage of development and they divide simultaneously. In a common batch-culture, the population contains cells in all possible stages of development, some have just been produced by fission, others are in intermediate stages and still others are mature and ready for division.
All these stages are parts of the cell-cycle. For this reason, the cell population of a batch culture divides asynchronously producing a typical exponential growth curve indicating that the logarithms of cell number as well as of cell-mass increase linearly with time (see Fig. 7.6).
In a synchronous culture, on the other hand, one would expect a step-wise increase in cell number, because there would be no increase in cell number between two successive divisions. But the cell mass would still show a linear increase with time, similar to that found in a batch culture. This is due to the fact that the newly born cells go on increasing in mass between two successive divisions, though their number does not increase (Fig. 7.11).
Synchronization of cell division in a bacterial population can be achieved in several ways. One of them is repeated alternation of the incubation temperature. A culture growing at the optimal temperature is exposed to a lower temperature, so that the growth is slowed down considerably. After some time, the culture is again brought to its optimal temperature.
The time interval is adjusted according to the growth rate (generation time) of the organism. By lowering the temperature, cell division is delayed and all cells divide simultaneously when the temperature is raised to the optimal level. The treatment has to be continued through several cycles to obtain satisfactory results. Another method of getting a synchronously dividing population of cells is by filtration through a membrane filter.
An asynchronous population of bacteria is filtered whereby the cells are adsorbed in the pores of the membrane filter. In the adsorbed state the cells continue dividing producing progeny cells which are not adsorbed. A reverse flow of sterilized medium through the membrane filter washes away the newly born progeny cells into the filtrate. Thus, a homogeneous population of cells which are approximately at the same stage of development can be obtained. Such cells divide synchronously for a few generations.
The utility of a synchronous culture lies in the fact that the whole culture can be taken as a multicellular complex consisting of individuals having identical development. In contrast, an asynchronous culture can give only an average picture about individual cells.
Sometimes, it becomes necessary to gain insight into the sequence of events taking place in individual cells. For smallness of size, study of such events in single bacterial cells is not feasible. A synchronous culture provides this opportunity, because all cells are in the same stage of development. For example, one might want to study the replication of DNA in relation to the cell cycle.
Samples withdrawn at suitable intervals during one unit generation time of a synchronous culture would yield cells at different stages of the cell cycle and each sample would contain cells of a particular stage. A measurement of incorporation of a radioactive DNA precursor would provide useful information of DNA synthesis at different phases of the growth cycle.
8. Culture Media:
Majority of bacteria and many eukaryotic microorganisms like algae and fungi can be cultivated under artificial conditions (as opposed to their natural habitats) on suitable culture media. A culture medium must contain all the raw materials that are needed by the particular organism to build up its cellular constituents — carbohydrates, proteins, lipids, nucleic acids etc.
Since water is the most important constituent of all living systems, microorganisms can best thrive in an aqueous medium. The main constituent of the culture medium is, therefore, water, in which the other ingredients are present in a dissolved state.
Microorganisms, like plant cells, are generally invested with a cell wall and they can take up nutrients only when they are in a dissolved state. The major barrier for entry of nutrients into the cell is, however, not the cell wall, but the cytoplasmic membrane. Some of the soluble nutrients can diffuse passively through the membrane, but for the majority there are specific transport systems located in the membrane which help in the uptake of nutrients from the culture medium.
The major elements that are essential for growth of all microorganisms are carbon, nitrogen, hydrogen, oxygen, phosphorus, sulfur, magnesium, calcium, potassium and iron. Many microorganisms also require traces of one or more minor elements, like manganese, molybdenum, zinc, cobalt, nickel, copper, boron, sodium and silicon.
All the elements required for growth have to be provided in the culture medium. Majority of bacteria and all fungi have a heterotrophic mode of nutrition and they are dependent on one or the other organic compound as source of carbon and energy. In general, microorganisms can take up all the other elements in inorganic form.
Thus, for growing the common bacteria and fungi present in soil or water, an inorganic salts medium containing a single organic compound proves adequate. But pathogenic bacteria often refuse to grow in such simple culture media and require supplementation of complex organic compounds, probably because they get accustomed to such compounds while growing in the body of the host. For example, Haemophilus requires haemin and nicotinamide adenine dinucleotide (NAD) in their growth medium.
Although common microorganisms (bacteria and fungi) can grow in an inorganic salt medium with a single carbon-source (generally glucose), they grow much faster when the medium is enriched by addition of some complex organic compounds.
On this basis, culture media can be distinguished into two types:
Complex and
Chemically defined.
The complex media contain one or more substances of undefined chemical composition. Commonly employed substances of this type are beef-extract, peptone, tryptone, yeast-extract, malt extract, blood, serum, egg albumin, potato extract, straw-infusion etc.
Casein-hydrolysate is another complex supplement, though its composition is more or less known. A complex bacteriological medium very commonly used for obtaining rapid and good growth is nutrient broth which contains beef-extract, peptone and sodium chloride.
Similarly, for growing saprophytic fungi, a common complex medium is malt-extract agar. Potato-dextrose agar — which contains extract of boiled potato, glucose and some salts — is another medium for growing fungi. Straw-infusion broth is a good complex medium for growth of soil actinomycetes. For growth of pathogenic bacteria blood agar, serum-albumin agar are often used. Among complex media are also to be counted some natural substances like milk, potato, carrot etc.
The chemically defined media, also called synthetic media, contain substances of known chemical composition and each of them is present in a known concentration. A simple synthetic medium which supports growth of common bacteria including Escherichia coli may be prepared from the following ingredients: K2HPO4 7.0, KH2PO4 3.0, Na3-citrate. 3H2O 0.5, MgSO4. 7H20 0.1, (NH4)2SO4 1.0, glucose 2.0, FeSO4 . 7H20 0.01, CaCl2 .2H20 0.01 (g/1). Similarly, for fungi, a synthetic medium is Czapek-Dox.
Culture media can be used in liquid form (broth) or in the form of a soft or a relatively hard gel (solid media). Gelatin which was used in the early days of microbiology as gelling agent is no longer in use except for special purposes. It has been completely replaced by agar-agar which is a much superior agent.
Agar-agar is a complex, highly cross-linked polysaccharide extracted from some marine red algae. At a concentration of 1.5-2% (w/v) it produces a solid gel, and at half the above concentration a soft gel. It sets to a gel at 42°-45°C and the gel can be melted at 100°C. The process of gelling and melting can be repeated several times, unless the pH of the medium is acidic.
Unlike gelatin, agar is not hydrolysed by most bacteria. The surface of agar media remains more or less dry, so that colonies appearing on the surface do not spread, and discrete colonies can be easily picked up. All these qualities make agar-agar an ideal agent for solidification of microbiological culture media.
There are some organisms, though very few, like Nitrosomonas which refuse to grow on agar media. For such highly fastidious organisms, the use of an inorganic gel becomes necessary, such as silica gel. It can be prepared by acidifying a solution of sodium silicate with hydrochloric acid. After setting, the gel is washed free of sodium chloride and excess acid. After drying the gel is allowed to absorb sterilized culture medium before inoculation.
Selective Media:
When a mixed population of different types of microorganisms is inoculated in a culture medium which allows selective growth of a single type or of a specific group, the medium is considered as selective. Selective media have to be designed according to the necessity for artificially increasing the number of a specific organism or, more commonly, for a specific group of organisms originally present in a mixed population (enrichment) or for direct isolation of a particular type from a mixed population.
A few examples of selective media can be cited. If one intends to study the types of nitrogen- fixing bacteria present in a certain soil, selective medium would contain other ingredients except any nitrogenous compound. In such a medium, organisms which are capable of utilizing atmospheric nitrogen alone would grow.
Again, if one wants to find out the number and select antibiotic resistant strains in a large population, the medium used for the purpose would contain the particular antibiotic at a concentration which is inhibitory for the sensitive strains. The nitrogen-free medium in the first case and the antibiotic-containing medium are examples of selective media.
Differential Media:
Whereas a selective medium permits the growth of a selective organism or a selective group of organisms, a differential medium allows growth of various types of organisms present in a mixed population, but at the same time helps to differentiate a particular organism or a group of organisms from the rest.
Thus in a mixed population, the presence of a particular type can be detected. For example, the presence of coliform bacteria in a water sample suspected to be contaminated with fecal matter can be detected by gas production from lactose. The ability to utilize a certain sugar can be similarly tested on a solid medium containing the particular sugar and a mixture of indicator dyes — eosine and methylene blue.
The organisms capable of fermenting the sugar to produce acid form colonies which absorb the dyes and produce a metallic sheen. Thus, on this differential medium (eosine-emthylene blue agar, EMB) the organisms can be visually differentiated from the rest. The endo-agar medium prepared by adding decolorized basic fuchsin in a lactose containing agar medium is another differential medium for identification of coliform bacteria.
9. Enrichment Culture:
Enrichment culture technique, developed by Winogradsky and Beijerinck, is based on the Darwinian principle of the survival of the fittest. In an enrichment culture, the environment is preset in such a manner that an organism or a group is selectively encouraged to grow and multiply, so that in a mixed population they become predominant.
The environmental factors that can be utilized for such selective growth or enrichment include carbon, nitrogen and energy sources, oxygen tension, pH, temperature, light etc. These factors have to be selected on the basis of the knowledge about the physiological-biochemical abilities of the organism desired to be enriched.
The question of enrichment arises when the desired organism needs to be isolated from a mixed population in which it forms a minority and is far outnumbered by other organisms. The usual dilution plating method cannot be employed as such, because during dilution the desired organisms are eliminated.
Therefore, before making dilution plates, the number of the desired organisms has to be increased preferentially over the non-desired organisms. In an enrichment culture the competition between the desired and non-desired organisms is removed or minimized, so that after few passages through enrichment culture, the desired organisms become the majority and then they can be isolated by the usual dilution procedure.
Success in such enrichment procedure naturally depends on how effective the selective conditions are. For creating such selective conditions, the knowledge about the desired organism is an essential prerequisite. The enrichment culture technique is a powerful microbiological tool which can be applied for isolation of any specific organism from its natural habitat, provided the selective conditions for its enrichment are known.
A few examples of application of the technique for isolation of different types of microorganisms may be cited:
Enrichment of di-nitrogen-fixing bacteria and endospore-forming bacteria:
If an inorganic salts medium without any nitrogenous compound and containing an organic compound as carbon and energy source is inoculated with a soil or water sample and incubated under aerobic conditions, the selective conditions thus created will encourage the growth of such organisms which can utilize the atmospheric nitrogen gas as the sole source of nitrogen.
Because the atmospheric nitrogen is in molecular form (N2), the organisms enriched are called di-nitrogen-fixers or diazotrophs. The little amount of combined nitrogen that is present in the soil or the water sample used as inoculum might allow growth of non-fixers at the initial stage, but a few passages through the selective media generally eliminate them.
The organisms that can be isolated in this way include different species of Azotobacter, Beijerinckia, Derxia, Azomonas etc. If a similar medium is used and incubated under anaerobic condition, it leads to enrichment of di-nitrogen-fixing anaerobes like Clostridium pastorianum and related species. Enrichment of Clostridia may be further facilitated when the inoculum is pretreated for 5 min at 80°C.
This treatment kills all bacterial vegetative cells, but endospores are spared. On the other hand, a mineral salts-sugar medium with combined nitrogen inoculated with a heat-treated inoculum incubated aerobically is suitable for enrichment of aerobic spore-forming bacteria, mostly different species of Bacillus. By selecting a higher temperature for incubation, it is possible to isolate the thermophilic species of this genus.
The conditions for enrichment of nitrogen fixers and spore-formers are summarised in Table 7.2:
Enrichment of Autotrophic Bacteria:
Autotrophic bacteria include the phototrophic and the chemolithotrophic organisms. The phototrophic prokaryotes include the anaerobic photosynthetic bacteria and the aerobic cyanobacteria. The chemolithotrophic bacteria similarly include a variety of organisms capable of oxidizing different inorganic substrates for obtaining energy.
All autotrophic organisms, including green plants, are able to synthesize organic compounds from CO2. For enrichment of autotrophic bacteria of any type, the media must not contain any organic compound, instead there should be a source of CO2, like bicarbonate.
For enrichment of all phototrophic organisms, the enrichment cultures must be exposed to light. Cyanobacteria are aerobic and many of them can fix atmospheric molecular nitrogen. For their enrichment a mineral salts medium without combined nitrogen, inoculated with a water or soil sample, should be exposed to light under aerobic conditions.
Cyanobacteria carry out oxygenic photosynthesis like green plants. The other group of phototrophic prokaryotes includes the anaerobic organisms which can be divided into two main types — the photolithotrophs and photo-organotrophs. For both, light is the source of energy for CO2-fixation, but whereas the photolithotrophs can use reduced sulfur compounds like H2S as electron-donor, the photo-organotrophs use simple organic compounds like acetate, malate etc. for this purpose.
Conditions for enrichment of different phototrophic prokaryotes are depicted in Table 7.3:
The chemolithotrophic bacteria, like nitrifying, sulfur-oxidising and hydrogen-oxidising bacteria are non-photosynthetic and strictly aerobic. They should be enriched preferably under dark condition to avoid growth of cyanobacteria. The enrichment medium should be composed of inorganic salts including a nitrogenous salt, and an inorganic oxidisable compound acting as energy source.
This compound will depend on the kind of organism to be enriched. For example, for nitrifying bacteria an ammonium salt can be used both as source of nitrogen and of energy. Another group of nitrifying bacteria use nitrite as energy source.
For both types, the enrichment medium is adjusted at an alkaline pH (8.5) and, furthermore, an insoluble acid-neutralizer like CaCO3 or MgCO3 has to be added to counteract the nitrous and nitric acid produced by the bacteria. Carbon dioxide released through interaction of acid and carbonate is helpful for growth. A number of passages through such media are required for adequate enrichment.
Another important group of autotrophic bacteria is the sulfur oxidizers. They can grow under autotrophic conditions using reduced sulfur compounds, like thiosulfate, sulfide or elemental sulfur and produce sulfuric acid. For their enrichment, a mineral salts medium including an inorganic nitrogenous compound and the oxidisable sulfur compound is used. The organisms are aerobic and can tolerate extremely acid pH, although they grow optimally at a neutral pH. Some species are obligately autotrophic and some others can grow also heterotrophically (facultative).
A third group of autotrophic bacteria comprises the hydrogen oxidizing organisms which utilize the oxidation of H2 to water as the energy-giving reaction. They are commonly known as hydrogen bacteria and all of them are only facultatively autotrophic. For their enrichment a mineral salts medium with combined nitrogen and without any organic carbon source contained in a closed vessel is generally employed. The gas phase of the vessel is artificially produced by replacing air with a mixture of H2 (70%), O2 (20%) and CO2 (10%) (v/v).
The conditions for enrichment of the nitrifying, sulfur oxidizing and hydrogen bacteria are shown in Table 7.4:
10. Requirements of Macro- and Micro-Elements for Growth:
These elements must be provided to microorganisms for growth. Water forms the major part of all actively growing living systems and microorganisms are no exceptions. But microorganisms— particularly endospores produced by some of them — can withstand desiccation for a long time without losing viability. Though, in such a state, their metabolic activities and growth are practically absent.
Besides water, carbon is the most important element that constitutes about 50% of the dry weight of bacterial cells. The importance of this element lies in the fact that carbon forms the skeleton of all organic compounds which are present in all biologically significant molecules, like those of proteins, carbohydrates, lipids, nucleic acids etc.
The majority of microorganisms have a heterotrophic mode of nutrition and they derive their, supply of carbon from one or the other organic compounds, like sugars, organic acids, alcohols, complex carbohydrates etc. All such heterotrophs can also fix small amount of CO2 into/several metabolic intermediates (heterotrophic CO2-fixation).
In contrast, the phototrophic and chemolithotrophic microbes draw their carbon requirement fully or for the most part from CO2. Some facultative autotrophs can grow both under lithotrophic (purely inorganic substrates) or under heterotrophic conditions. Some have a mixed type of nutrition, the mixotrophs which can simultaneously utilize CO2 and some organic compound as carbon source.
Next to carbon is nitrogen which forms about 10-15% of the dry weight of microbial cells. Most bacteria can grow only in the presence of combined nitrogen supplied in the medium. A few, including some cyanobacteria, can reduce molecular nitrogen to ammonia and incorporate it into organic acids to produce amino acids.
This property, known as di-nitrogen fixation, is conferred by a special enzyme complex known as nitrogenase. Nitrogen is present in proteins, nucleic acids, cell wall polymers, coenzymes, vitamins etc. Among these, proteins are quantitatively the most important accounting for about 50% of the dry weight of bacterial cells.
Phosphorus is the next major element forming 2-6% of the dry weight of bacterial cells. Microorganisms get their supply of this element from inorganic phosphates provided in the growth medium. Phosphates also help to keep the pH of the medium in a favourable range by a buffering action.
Among the most important cellular constituents that contain phosphorus are the nucleic acids. DNA accounts for 3-4% of the dry weight of bacterial cells and RNA for 10-20%. The energy-rich compounds, like adenosine triphosphate (ATP), guanosine triphosphate (GTP) etc. and phosphorylated sugars are also among important phosphorus containing compounds. Besides, phospho-lipids form the membrane system.
Sulfur is also an essential element for all living organisms. It is found in all proteins as a constituent of the amino acids, cysteine and methionine. Two cysteine molecules can join by oxidation to form a dimer, called cystine (S-5 bond, disulfide bridge).
This reaction is of special significance in imparting a characteristic folding of the polypeptide chain, and also it plays an important role in joining individual polypeptide chains to produce the quaternary structure of protein molecules. Microorganisms get their supply of sulfur from inorganic sulfates present in medium.
Sulfate is reduced intracellularly by an assimilatory reduction pathway to HS– (sulfhydryl) and incorporated into organic compounds. The element sulfur has special significance in case of sulfur-oxidizing chemolithotrophs, like Thiobacillus and the purple and green photosynthetic sulfur bacteria, like Chromatium and Chlorobium.
Some species of Thiobacillus can oxidize elemental sulfur to sulfate and utilize the oxidation energy for chemolithotrophic growth. Purple and green sulfur bacteria utilize sulfide as exogenous electron donor in photosynthesis and in the process they produce elemental sulfur which deposits within or outside their cells.
So far we have considered the role of non-metallic elements in the constitution of microbial cells. Though quantitatively less, metallic elements also form essential parts of microorganisms. Among them, magnesium (Mg++) and potassium (K+) are required in substantial amounts. Mg++ is essential for maintenance of the integrity of ribosomes and it acts as a co-factor in many enzyme reactions, particularly those involving ATP.
Moreover, it is present as a part of chlorophyll in all photosynthetic organisms, including cyanobacteria, and purple, green and non-sulfur photosynthetic bacteria containing bacteriochlorophylls. Potassium (K+) is one of the important intra-cellular elements for maintenance of ionic balance. It also serves as a co-factor in many enzyme reactions and it plays an important role in ribosomal function.
Iron (Fe++) forms an integral part of all haem-proteins, like cytochromes. It is also present in ferredoxin and nitrogenase. Iron bacteria, like Thiobacillus ferrooxidans, are able to grow chemolithotrophically at the expense of the energy of oxidation of Fe++—>Fe+++. Among other metallic elements required by microorganisms are zinc (Zn++), molybdenum (Mo++), manganese (Mn++), calcium (Ca++), cobalt (Co++) and nickel (Ni++).
Zinc and manganese act as cofactors for several enzymes. Molybdenum, together with iron, are integral parts of the enzyme nitrogenase. Another molybdenum enzyme is dimethyl sulfoxide reductase which converts the sulfoxide to dimethyl sulfide. The enzyme has an important role in the sulfur-cycle of nature. Nitrate reductase is still another molybdoenzyme.
Some nitrogen-fixing bacteria possess nitrogenase in which vanadium (Va++) replaces molybdenum. Nickel (Ni++) is required by hydrogen-oxidising bacteria for hydrogenase activity. Some bacteria can synthesize cyanobalamine (Vitamin B12) and they require cobalt (Co++) which is present in the vitamin. Calcium (Ca++) is essential for production of endospores in Gram-positive bacteria. The endospores contain calcium dipicolinate, a compound which is largely responsible for thermo resistance.
Zinc and manganese ions serve as co-factors in several enzyme reactions. Sodium ion (Na+) is not generally required by most bacteria, although it is sometimes added in culture media mainly for maintenance of a favourable osmotic pressure of the medium.
Most of the common bacteria can tolerate a moderate concentration of NaCl (3-4%), but the marine microorganisms require a higher concentration for growth. There are some extremely salt-tolerant bacteria (the halophiles) like Halobium which need a much higher concentration of NaCl (up to 20%) for maintaining their cell integrity.
Average elemental composition of dry bacterial cells is shown diagrammatically in Fig. 7.12:
11. Physical Factors Influencing Growth:
(i) Oxygen:
On the basis of oxygen relation, microorganisms are classified into three major types — aerobic, anaerobic and microaerophilic. While aerobic organisms require oxygen of air for growth, anaerobic organisms — which include mainly bacteria — are unable to utilize oxygen. For some obligate anaerobes oxygen is even toxic. Some organisms which are able to grow both in presence of air or in its absence are called facultative anaerobes. The microaerophilic organisms are also aerobic, but they grow only at a reduced oxygen tension.
The aerobic organisms are capable of oxidizing substrates fully to CO2 and H2O using oxygen as the terminal hydrogen acceptor. In this process of respiration they produce ATP in the electron transport system by oxidative phosphorylation from ADP and inorganic phosphate.
The obligate anaerobes, on the other hand, can oxidize substrates only partially by fermentation, because of the lack of tricarboxylic acid cycle (TCA cycle)-linked electron transport pathway and they produce ATP only by substrate level phosphorylation.
The facultative anaerobes are essentially of two types. Some are capable of changing their metabolism either to fermentation or to aerobic respiration depending on the environmental conditions i.e. whether oxygen is absent or present.
The other group of facultative anaerobes are respiring organisms capable of utilizing oxygen either from air (when air is present), or from oxidized inorganic compounds, like nitrate or sulphate (when air is absent i.e. under anaerobic conditions).
Under anaerobic conditions they carry out the so-called nitrate or sulphate respiration, in which these compounds serve as the terminal electron acceptor in place of oxygen. They produce ATP by oxidative phosphorylation like aerobic organisms.
Microaerophiles are also oxygen-requiring respiring organism, but they can grow only when the oxygen concentration is considerably lower than normal. This is probably due to the oxygen sensitivity of some of their vital enzymes. In an un-agitated liquid culture these bacteria form a layer below the surface where the oxygen concentration is suitable for their growth. In contrast, the aerobic organisms grow at the surface and the facultative anaerobes tend to accumulate at the bottom, if they grow at all.
Bacteria can utilize oxygen dissolved in the growth medium. The solubility of oxygen in water is low and most bacteria are well-adapted to such concentration for normal growth when they grow on agar surface or at the air-liquid interface.
Use of thin layers of culture medium in vessels with a wide surface generally suffices for normal growth of most aerobic organisms. However, when use of larger volumes of medium becomes essential, need for additional arrangement for aeration arises.
For laboratory flask cultures, a mechanical shaker having either a to and fro (reciprocal) or an elliptical movement is commonly used for agitating liquid cultures. When still larger volumes have to be used, arrangements for forced aeration become necessary.
Generally, for this purpose, sterilized air is forced through a sparger producing air-bubbles at the bottom layer of the culture fluid. Larger fermenters are provided with more sophisticated arrangements for aeration. For growing anaerobic organisms, oxygen has to be excluded from the culture medium and the atmosphere existing in the culture vessel. Some anaerobes are somewhat aero-tolerant and they can grow in solid media containing reducing substances like thioglycollate, ascorbic acid or cysteine which presumably minimize the toxic effect of oxygen by interacting with it.
For growing anaerobic organisms in liquid culture, generally closed vessels filled completely with the freshly prepared medium are employed. If an artificial atmosphere (gas-phase) is necessary, it must be oxygen-free. Specially designed containers (anaerobic jars) are available for growing anaerobes.
(ii) Hydrogen-ion concentration:
Hydrogen-ion concentration [H+] determines the acidity and alkalinity of the culture medium and is commonly expressed as its pH value which is logarithm of the reciprocal of hydrogen ion concentration (gram molecules per liter). The hydrogen ion concentration of pure water is 1/107 moles per liter corresponding to a pH value of 7.0 (neutral). As [H+] increases pH value falls and acidity increases. Decrease of [H+] from the neutral point results in increase of alkalinity.
The relationships are shown in Fig. 7.13:
ADVERTISEMENTS:
Most bacteria prefer a neutral to slightly alkaline medium, while fungi and algae grow better at slightly acidic conditions. For each organism, there is a minimum, an optimum and a maximum pH, permitting growth. The total range may be quite broad extending over 2-3 pH units which corresponds to a difference of 100-1,000-fold in hydrogen-ion concentration. In spite of this wide variation, organisms are able to grow because the cell membrane is hardly permeable to H+ or (OH)–. As a result, the cell interior remains more or less neutral.
Microorganisms during growth may produce acidic or alkaline substances in considerable quantities to change the pH of the medium to such an extent which proves inhibitory. For example, the nitrifying bacteria produce nitrous and nitric acids as oxidation products and they turn the medium highly acidic and unsuitable for growth. An opposite situation prevails in case of ureolytic or proteolytic bacteria. They produce ammonia as the end-product which turns the medium strongly alkaline.
For checking these extreme and unfavorable changes in the pH of the culture media, they have to be adequately buffered. Generally, the acidic and alkaline phosphates, like KH2P04 and K2HP04, are used in bacteriological media. An equimolar solution of these two salts produces a pH of 6.8 which is suitable for growth of many bacteria.
If an organism produces an acid in the medium, it reacts with the alkaline phosphate turning it to the acidic form. Opposite is the case when an organism tends to change the medium alkaline by producing a basic substance. Thus, phosphates which are well tolerated by most bacteria are widely used for buffering. At the same time they serve as source of phosphorus.
Though buffering of the medium is generally found to be adequate for checking pH shifts, sometimes, as in the case of nitrifying bacteria which produce profuse amounts of acids, addition of insoluble neutralizers like calcium or magnesium carbonates becomes necessary.
Bacteria, in general, prefer a neutral to slightly alkaline growth medium. But there are exceptions also. The bacteria growing optimally at an acidic pH are called acidophils and those growing preferentially in an alkaline pH are known as alkalophiles.
Not only bacteria (both eubacteria and archaebacteria) but also a number of algae, fungi or even flagellates are endowed with such properties. Many of the acidophilic organisms are simultaneously thermophilic. Some examples of acidophilic bacteria are Acetobacter acidophilum (optimum pH 3), Thiobacillus thiooxidans (pH range for growth 0.9-4.5), Bacillus acidocaldarius (pH range for growth 2-6, opt. 3), Thennoplasma acidophilum (pH 1-2), Sulfolobus acidocaldarius (pH range 1.5-3.5), Non-bacterial organisms which can grow in an acidic pH include Cyanidium caldarium (eukaryotic alga, pH 2-3), Chlorella ellipsoidea (pH 2), Chlamydomonas acidophila (pH 2), Polytomella caeca (pH 1.4 — a flagellate). Some fungi, like species of Cephalosporium, Trichosporon, Aspergillus, Penicillium and Fusarium, can also grow at considerably acidic media.
The true alkalophilic organisms have pH range for growth varying between 8-11 or even more. They include some blue-green algae e.g. Spirullina and Synechococcus, true bacteria, like Bacillus alkalophilus and several other bacilli and some archaebacteria, like Natronobacterium sp., Methanobacterium thermoalcalophilum etc.
(iii) Temperature:
Temperature is one of the most important variables for growth. For every organism exists a temperature range which is congenial for its growth. At one extreme of this range is a minimum temperature where growth can start and at the other extreme is the maximum where growth abruptly stops. In between the two extremes, there is an optimum temperature where growth rate is maximum.
On the basis of temperature relations, microorganisms are classically divided into three major groups — psychrophiles, mesophiles, and thermophiles. Psychrophiles are cold-loving organisms growing in marine or alpine habitats. Many of them can multiply at 0°C or even sub-zero temperature and have maximum growth rate between 16°C and 20°C.
Well-known examples of psychrophilic bacteria are marine Photo-bacterium sp. and the iron-oxidizing Gallionella ferruginosa (opt. temp. 6°C). Besides, several marine species of Pseudomonas and some species of Bacillus isolated from glaciers belong to this group.
The majority of bacteria and other microorganisms belong to the mesophilic group having an optimum temperature between 20°-45°C. Growth of most mesophiles stops abruptly between 45°- 50°C. Organisms which can grow at higher temperatures are called thermophiles. Several types of thermophilism can be noticed. Organisms which grow optimally above 65°-70°C and are unable to grow below 40°-45°C are called obligate thermophiles. Organisms which grow at mesophilic temperatures but can survive exposure to 60°-65°C are called thermo-tolerant (e.g. Methylococcus capsulatus).
Organism having a maximum temperature of growth at 50°-65°C, but can grow also at mesophilic temperature are called facultative thermophiles (e.g. Bacillus coagulaus). Among the obligate thermophiles some can grow at temperature ranging between 80°-100°C. They are called hyper-thermophiles. In more or less recent times, a good number of such organisms have been discovered e.g. Thermotoga maritime (max. temp, of growth 90°C), Aquifex pyrophilus (max. 95°C), Desulfurolobus ambivalens (max. 95°C), Pyrobaculum aerophilum (max. 104°C), Pyrococcus furiosus (max. 100°C), Pyrodichtium brockii (110°C), Hyperthermus butylicus (max. 108°C) etc.
Majority of the hyper-thermophiles except the species of the genera Thermotoga and Aquifex are archaebacteria. Interest in the hyper-thermophilic organisms has greatly increased in recent times due to the possible presence of thermo-stable enzymes in them e.g. the thermo-stable DNA polymerase from Thermus aquaticus, technically called Taq-polymerase, is used in PCR.