ADVERTISEMENTS:
Read this article to learn about the atmospheric pollutants:- 1. Sources of Atmospheric Pollutants and 2. Effects of Atmospheric Pollutants.
Sources of Atmospheric Pollutants:
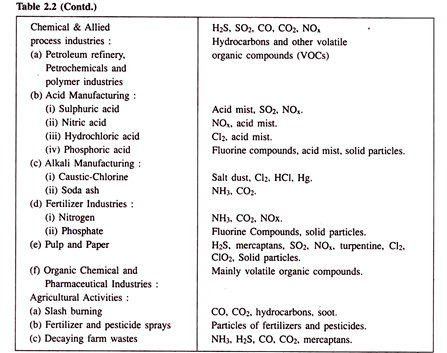
Pollutants are emitted to the atmosphere as a result of natural processes as well as due to human activity. The sources of some common pollutant are tabulated in Table 2.2. It should be pointed out here that the list is not a complete one. Natural sources of emission are oceans, volcanoes, swamps, biologically decaying organic matter, desert and semi-desert areas, forests and forest fires, lightning, etc.
Human activities, which give rise to air-borne pollutants, are domestic, transport, agricultural and industrial. The major industrial sources are fossil fuel combustors, mines, quarries, metallurgical and metal processing industries, chemical processing industries; food, biochemical and pharmaceutical industries.
ADVERTISEMENTS:
Effects of Atmospheric Pollutants:
Air pollutants affect ecosystems in various ways. The effects are manifested by bringing about some changes in an ecosystem directly or indirectly.
ADVERTISEMENTS:
The overall effects may be classified as hereunder:
I. Effects on atmospheric properties
II. Effects on vegetation’s
III. Effects on animals
IV. Effects on human beings
V. Effects on land and water bodies
VI. Effects on materials.
I. Effects on Atmospheric Properties:
The atmospheric properties of the troposphere get affected considerably due to the presence of pollutants and those of the stratosphere to some extent. The properties of the other layers (mesosphere and thermosphere) remain almost unaffected, as pollutants are virtually not present there.
A. Tropospheric Effects:
ADVERTISEMENTS:
The gaseous and particulate pollutants which are directly emitted into the troposphere due to natural as well as human activity affect the following properties of the troposphere:
(i) Visibility,
(ii) Fog and haze formation,
(iii) Precipitation,
ADVERTISEMENTS:
(iv) Solar radiation incidence,
(v) Temperature,
(vi) Wind direction and velocity.
(i) Visibility:
ADVERTISEMENTS:
When a beam of light passes through air its intensity decreases due to absorption and scattering by the molecules of gases present in air as well as by the suspended particles (particularly by the sub- micron sized ones) present in air.
The major constituents of air, namely, oxygen, and nitrogen do not absorb or scatter light, but the pollutant gas molecules and suspended particles (both liquid and solid) do. The extent of absorption and scattering is dependent on the specific pollutants present and their concentration.
Decreased intensity of light as a result of absorption and scattering decreases visibility. Nitrogen dioxide and aerosols strongly absorb visible light in the blue-green wave length range and produce a yellow-brown haze. Ozone absorbs at wave lengths below 3200 A.
Hygroscopic particles present in air picks up moisture from air as the humidity increase and consequently reduce the visibility further. At a humidity of 70% R. H. and less the effect is not that marked, but above 70% R. H. the effect is considerable.
ADVERTISEMENTS:
Visibility being dependent on the concentration of the gaseous pollutants and suspended particles is influenced by the wind speed. With the increase in wind speed up to about 24 kmph the visibility improves as the pollutant concentration decreases due to dispersion. But at higher wind speed, due to scouring effect the concentration of solid particles (dust) increases in air. This leads to more scattering, and consequently visibility becomes poorer.
(ii) Fog and Haze Formation:
Polluted air having a large number of suspended particles is conducive to fog formation under favourable condition. Normally air is unsaturated with water vapour. When air temperature decreases (at night) it may become saturated and with further decrease in temperature air may become supersaturated with water vapour. Under such condition a suspension of fine water droplets in air, that is, a fog is formed. Fine suspended solid particles present in air serve as condensation nuclei.
If the number of particles be large, the water droplets formed would be small in size and the fog would be more stable. Such a fog would be more effective in scattering light and thereby reduce visibility. If fewer suspended particles be present in air the water droplets would be larger in size, hence the resulting fog would be less stable.
When a large number of tiny solid particles are present in air, they absorb and scatter light. This results in reduced visibility. Such a situation is termed as haze. Fine solid particles may get suspended in air due to high wind velocity and or due to emission from industrial units as well as due to aerosol formation.
(iii) Precipitation:
ADVERTISEMENTS:
When the relative humidity (RH) in air becomes sufficiently high the nucleating particles get ‘activated’ and condensation of water vapour in atmosphere is initiated. The mechanism by which the cloud condensation nuclei (CCN) act is not known; however, it is known that hygroscopic and water soluble particles are more effective as CCN. The sources of CCN may be natural processes as well as industrial operations. The CCN emitted from industrial sources supplement the natural CCN.
Once the process of condensation is initiated further development would depend on the number and size of the droplets in a cloud. If there are a larger number of smaller droplets, those would not agglomerate easily and hence there would be less precipitation. If the number of CCN be relatively fewer, the size of droplets produced would be larger which would coalesce more readily and thereby would cause more precipitation. The precipitation may be either dry (snow) or wet (rain) depending upon the ambient air temperature.
(iv) Solar Radiation Incidence:
Pollutants present in air interfere with the incidence of solar radiation on the earth’s surface by scattering and absorbing the incoming radiation. Solid and liquid particles generally scatter the incoming radiation whereas the gaseous pollutants and aerosols absorb radiation.
Absorption occurs at specific wave lengths depending upon the pollutants present. For example, nitrogen dioxide absorbs radiation in the wave length range of 3600-4000 A, whereas ozone absorbs at wave lengths below 3200 A.
Due to scattering and absorption of incoming solar radiation by air-borne pollutants less energy would reach the earth’s surface resulting in lower surface temperature. The energy absorbed by gaseous pollutants is released to the atmosphere as heat which causes rise in tropospheric temperature.
ADVERTISEMENTS:
Cloud cover also reduces incidence of solar radiation on the earth’s Surface.
(v) Temperature:
During the daytime solar radiation reaches the earth’s surface, a part of which is absorbed. Subsequently the ground transfers a portion of the absorbed radiation to the tropospheric air by re- radiation and convection. At higher altitude of the troposphere the flux due to re-radiation and convection is less.
Consequently the air temperature is less. The variation of temperature with altitude in the troposphere is also due to the decrease in atmospheric pressure with altitude. This decrease is due to adiabatic expansion of ‘air packets’ as they move upward because of convection.
The intensity of solar radiation at a place in the ground level depends on the angle of incidence, which in turn depends on the time of the day (relative to sun rise) as well as on the season. On a cloudless day and the air free from pollutants, the intensity is maximum at mid-day when the sun is overhead. When pollutants are present at a higher concentration in air, and there is cloud cover, the solar intensity is less. Variation of solar intensity results in changing the normal tropospheric temperature profile.
The ground receives solar radiation and re-radiates a part of it during the daytime but at night it re-radiates only. Hence the ground temperature decreases gradually after sunset and after some point of time it becomes less than that of air above it. This situation is termed as ‘inversion’.
Normally after sunrise the ground starts receiving solar energy and its temperature increases gradually, which results in the disappearance of the inversion condition. However, when pollutants are present in air at higher concentration the state of inversion may continue for a longer period even after sunrise because of lesser incidence of solar radiation. In severely polluted atmosphere the inversion may continue for days together.
Vertical convective movement of air in the troposphere occurs due to its negative temperature gradient. Such movement helps in dispersing the pollutants. The factors mentioned earlier affects the ‘normal’ tropospheric temperature gradient and thereby interferes with the pollutant dispersion process.
The tropospheric temperature profile patterns under different atmospheric conditions are shown in Fig. 2.2. It should be noted here that the atmospheric condition is termed as ‘stable’ when it resists the vertical convective movement.
A. Neutral:
The lapse rate is nearly identical to the dry adiabatic lapse rate. Such a condition prevails on days of moderate solar incidence. When some vertical air movement takes place the situation is referred to as neutral stable.
B. Super-Adiabatic:
The lapse sate is more than that of the dry adiabatic. On days of strong solar incidence such a condition is observed.
C. Sub-Adiabatic:
The lapse rate is less than that of the dry adiabatic. It happens on days of slight solar incidence. When very little vertical air movement takes place the condition is termed as slightly stable.
D. Inversion:
Air temperature increases with height up to some altitude and then decreases. Such a situation may occur at late night. Stable inversion occurs when there is no vertical air movement below the break. When the tropospheric condition is neutral, a rising ‘packet’ of air would undergo cooling due to adiabatic expansion and its temperature would be the same as that of the ambient air at that height.
As there would be no difference in density between the rising packet and the ambient air, further vertical motion of the packet would neither be suppressed nor be enhanced.
Under super adiabatic condition a rising packet of air even after cooling due to adiabatic expansion would attain a temperature higher than that of the ambient air at that height. Consequently, it would move further up. Such movement promotes vertical convection.
Under sub-adiabatic or inversion condition as a ‘packet’ of air moves upward due to turbulence, it becomes cooler and denser (because of adiabatic expansion) than the ambient air at that height and hence it tends to slide back. As a result the vertical motion is impeded.
The pollutants present in air not only cause tropospheric temperature profile change but may also cause increase in the average global temperature of the lower troposphere. Some gases, such as carbon dioxide, methane, nitrogen oxides and chlorofluorocarbon (CFC) absorb ground re-radiation (the infrared portion).
With an increase in concentration of the above-mentioned pollutants in air they will trap more of the ground re-radiation and thereby cause increase in the average global temperature of the troposphere. With the rise in tropospheric temperature, the earth’s surface temperature will increase because of heat exchange between them.
This effect is somewhat similar to what happens in a glass greenhouse. In a greenhouse the ground re-radiation is prevented from escaping to the space by a glass enclosure. As a result the temperature inside a greenhouse is always higher than that of the outside. Since the gaseous pollutants mentioned earlier cause waning of the troposphere, in the same way as in a greenhouse, those gases are referred to as greenhouse gases.
Carbon dioxide, which, as such, is considered to be non-polluting, is the major contributor to the greenhouse effect. The concentration of carbon dioxide in the troposphere is the highest compared to those of the other green-house gases.
Moreover, its concentration in the troposphere has been observed to increase at the rate of 3.8 percent per decade since 1970s because of progressive uncontrolled combustion of fossil fuels by man in order to meet his ever-increasing energy need.
The situation is getting aggravated because of deforestation. In view of the above, it is now universally accepted that there is no other alternative but to reduce the emission rate of CO2 particularly from the conventional fossil fuel based power plants.
Serious efforts are presently being made to develop carbon dioxide capture and storage processes as well as processes for converting captured CO2 to algae, methanol, hydrocarbons, etc. Progressive global warming resulting from greenhouse effect is likely to bring about various changes in the biosphere, such as, change in precipitation, decrease in agricultural production and melting of some of the permanent ice packs. If some of the permanent ice packs (glaciers and polar ice) melt, then the ocean level will increase, as a result of which the low-lying coastal areas of the earth would be inundated.
In order to save the earth (the only habitat of man) from not so far away catastrophic situation, the representatives from 160 countries met in Kyoto in 1997 to frame a protocol, which is known as Kyoto Protocol. The objective of the protocol is to make an all-out effort to reduce the rate of emission of the ‘greenhouse gases’.
The target fixed is to reduce the rate of emission progressively, so that by 2012 their concentration in the atmosphere would be 5.2% less than that in 1990. However, because of non-endorsement of the protocol by some of the developed countries there is every doubt that the target would be realized.
(vi) Wind Direction and Velocity:
Wind is generally defined as air movement in the horizontal direction. Vertical movement of air is referred to as updraft or downdraft.
The factors which influence motion of air in the horizontal direction are:
(i) The pressure gradient,
(ii) The Coriolis force, and
(iii) The frictional force.
The pressure gradient occurs mainly due to uneven rate of heating of land and ocean. Normally during the daytime the land mass gets heated quickly whereas the ocean heats up slowly. As a consequence, pressure gradient develops from ocean to land and sea breeze blows landward.
At night land cools quickly compared to the ocean and land breeze blows seaward. The wind speed depends on the magnitude of the pressure gradient, which in turn is influenced by the intensity of solar radiation, pollutant concentration in air, cloud cover and local topography.
The Coriolis force is caused by the rotation of the earth around its axis. This force deflects wind towards the right and it is proportional to the wind speed. The frictional force opposes movement of air. This force is maximum at the earth’s surface, hence the wind speed at any instant of time is the minimum at the ground level and it increases with the altitude.
The wind speed is generally measured at meteorological stations at a height of 10m. The speed at any other height is calculated using the relation.
Uh/U10 = (h/10)n
Where Uh = wind speed at a height of h in m,
U10 = wind speed at a height of 10 m,
n = an exponent, a positive fraction, 0.5 or less. Its numerical value depends on the atmospheric stability and the local features including its relief (nature and man-made topography).
In the absence of any specific information the following values may be taken depending on the weather condition:
n = 0.2 for unstable conditions,
= 0.25 for neutral conditions, and
= 0.5 for stable conditions.
The wind speed may be expressed in the unit of m/s or kmph.
The meteorological stations not only report wind speed but also its direction throughout the year. Such data are often presented graphically. Both the frequency and speed of wind in all possible directions (8 to 16) are drawn to a scale normally for a period of one year. Such a diagram is termed as a wind rose. Figure 2.3 shows a typical wind rose for Kolkata City.
The length of a bar segment of a wind rose represents its frequency from a particular direction, while the width/shade of the same represents the range of wind speed from that direction. Such a diagram is accompanied by a scale for the wind speed.
From the discussion so far it is apparent that the prime factor which gives rise to wind is the pressure gradient arising out of unequal heating and cooling of land and ocean. The other factors, which influence wind speed and direction, are the weather condition, the season, the ocean current, etc.
It should be noted here that high wind speed helps to disperse particulate matter and gaseous pollutants, but too high a wind speed scours dusts (during dry season) and thereby increases the solid particle concentration in air.
Particulate matter and gaseous pollutants when present in air at a relatively higher concentration interfere with the incidence of solar radian to the ground, as a result of which the wind speed is adversely affected. This in turn retards the process of dispersal of pollutants.
II. Effects on Vegetation:
The pollutants present in the troposphere affect vegetation in three different ways:
(i) By attacking the cells of the different parts of plants,
(ii) By interacting with enzymes present in plants and
(iii) By interfering with the photosynthesis process.
The effects may be visible or invisible.
The visible injuries to plants may be acute or chronic. Acute injuries occur when plants are exposed to a higher concentration of pollutants. The cell membranes may get ruptured due to chemical actions of gaseous pollutants resulting in loss of cell content and finally cell death.
Pollutants such as SO2, O3, PAN bring about such changes. Pollutants like SO2, NH3, O3, fluorides, and PAN affect chlorophyll, which is the key component for photosynthesis. As a result the leaves wither. Abnormal growth of leaves and stems are induced by ethylene and herbicides. Chronic visible injuries occur due to prolonged and repeated exposure of plants to pollutants at a relatively low concentration.
Invisible damages are manifested in the form of reduced growth, interference with photosynthesis and related processes. Reduction in fruit yield due to attack on the reproductive structure also takes place. SO2, O3, and fluorides adversely affect the growth rate. SO2, NOx, PAN, O3, and fluorides interfere with the photosynthesis process and metabolic pathways. Fluorides, O3 and other oxidants attack the reproductive structure.
Dust particles interfere with the photosynthesis process by covering leaf surfaces and also by blocking the stomata’s. The extent of damage to vegetation in polluted atmosphere depends on the plant species, the specific pollutants present, their concentration and the duration of exposure.
The injury threshold concentrations of some common pollutants are listed in Table 2.3.
III. Effects on Animals:
Fish and domestic animals get affected when they ingest toxic chemicals and heavy metals. Domestic animals may get affected in the same way as human beings by inhaling air-borne gaseous pollutants and particles.
Heavy metals, such as arsenic, lead, molybdenum, mercury and their compounds are emitted during industrial processes, like roasting, smelting, steel making, etc. These may accumulate on vegetation and forages which when consumed by herbivores may affect them. Man may get affected by consuming milk and meat of the affected herbivores.
IV. Effects on Human Beings:
Air-borne gaseous pollutants and suspended particles affect human health and may produce various types of physiological effects. Common pollutants, such as CO, SO2, NOx, hydrocarbons and particulates are directly emitted from industrial sources, whereas, O3, PAN, and some other oxidants are produced due to secondary reactions.
Various other toxic chemicals are emitted and are present in the surroundings of the places where those chemicals are produced, used or handled. The biological/physiological effects of some of the above mentioned pollutants are briefly discussed hereunder and are summarized in Table 2.4.
(i) Effects of Carbon monoxide (CO):
CO is a colorless and odorless gas. It affects by combining with hemoglobin (Hb) in blood. Hemoglobin carries O2 from the respiratory system to the different parts of human body and removes CO2 from those places by forming unstable complexes with O2 and CO2.
The affinity of CO towards hemoglobin is more than that for oxygen. As a result when CO is breathed in, it forms a relatively more stable complex (COHb, carboxyhaemoglobin) with hemoglobin; consequently the oxygen carrying and CO2 removal capacity of blood, decrease.
The level of COHb complex in the blood of a person depends on CO concentration in air as well as on the duration of exposure of an individual. If about 0.5 to 2% of the total hemoglobin is complexed with CO there would not be much effect. However, with increasing concentration of COHb in blood, progressively manifested symptoms are headache, dizziness, nausea, vomiting, and difficulty in breathing, collapse, unconsciousness and death.
The reaction between CO and hemoglobin being a reversible one when a person leaves a polluted area with a non-lethal dose of CO he breathes out CO and recovers. CO does not leave any permanent effect. The global average concentration of CO in air is about 0.1 ppmv. Its threshold limit is 50 ppmv. At a concentration of 1000 ppmv or more it is fatal.
(ii) Effects of Sulphur dioxide (SO2):
SO2 combines with water in the respiratory system to produce sulphurous acid (H2SO3) which irritates conjunctiva, upper respiratory tract and throat resulting in airway resistance. When inhaled at a concentration of 5 ppmv or more for about 10 minutes or so the pulse and breathing rate increase.
Symptoms such as nasopharyngitis and coughing develop on prolonged exposure. SO2 combined with benzo (a) pyrene (C2OH12), a product of incomplete combustion, causes pulmonary cancer. The global average concentration of so2 is 0.0002 ppmv. Its threshold limit is 2 ppmv. At a concentration level of 500 ppmv it is fatal.
(iii) Effects of NOx:
Nitric oxide (NO) and nitrogen dioxide are the major nitrogen oxides present in air. Of these two NO is less toxic. Like CO, NO combines with hemoglobin and thereby interferes with the oxygen transfer process. NO gets oxidized to NO2 which on inhalation is converted to nitrous acid (HNO2) and nitric acid (NHO3) in the lungs.
These exert toxic effects on the deep lungs and peripheral airway. At a lower concentration of NOx, the eyes, throat and lungs get irritated. Regular exposure at 10-40 ppmv concentration may cause distention of lungs and scarring of pulmonary tissues. Continued exposure at 500 ppmv or more would lead to death.
The threshold value of NO2 is 5 ppmv.
(iv) Effects of Ammonia(NH3):
NH3 is a colorless gas with a pungent odour. It is an irritant to skin, respiratory tract, mucous membranes and eyes. At higher concentrations it corrodes tissues and causes laryngeal and bronchial spasm and edema. At a concentration of about 5 ppmv it can be detected by its pungent smell.
At concentration around 150-200 ppmv it causes general discomfort and tears in the eyes. When a person is exposed to about 2000 ppmv concentration of ammonia, suffers from burns and skin blisters and progressively experiences serious edema, asphyxia and finally death.
(v) Effects of Hydrogen Sulphide (H2S):
H2S is a colorless gas with a rotten egg smell. It may cause eye irritation at a concentration of about 100 ppmv. Inhalation of H2S at a higher concentration irritates the entire respiration tract.
(vi) Effects of Chlorine (Cl2):
CI2 is a greenish yellow gas with a characteristic pungent smell. It irritates eyes, skin and the respiratory tract. At higher concentration of Cl2 one suffers from skin burns, redness of eyes, blurred vision and lung damage. At a concentration of about 1000 ppmv it becomes lethal.
(vii) Effects of Ozone(O3)
Inhalation of ozone above its normal concentration causes pulmonary inflammatory response. Exposure to ozone at a concentration of 1.5 to 2 ppmv irritates eyes, throat and lung. Continuous or intermittent exposure to ozone may cause chronic bronchitis, bronchiolitis and fibrotic changes of lungs. Ozone is a potential mutagen as it degenerates chromosome in lymphocytes. Its threshold limit value is 0-1 ppmv. Other photochemical oxidants present in polluted air, such as peroxyacetyle nitrate (PAN), peroxybenzoly nitrate cause effects similar to those of ozone.
Threshold Limit Value (TLV):
The Industrial Threshold Limit Value set by the American Conference of Governmental Industrial Hygienists (ACGIH) refer to indoor air-borne concentration of substances and represent the conditions under which it is believed that nearly all healthy adult workers may be exposed eight hours a day, five days per week without adverse effects.
Effects of Suspended Solid Particles (SSP):
Air-borne particles enter the respiratory system and get deposited in the different regions of the respiratory system depending upon their size, shape and density. The large (5 pm and more) and denser particles are deposited in the nasal region and are removed by mucous discharges. Finer and lighter particles penetrate deeper into the system.
Particles about 2 pm in size and finer enter into the lungs. Finer particles having larger external and internal surface areas (per unit mass) adsorb gaseous pollutants present in air and carry them deep inside the lungs. Particles with adsorbed pollutants cause more harm. The particles themselves may be toxic. The effects of some air-borne gaseous pollutants on human beings and their TLV are summarized in Table 2.4.
TWA:
Time Weighted Average concentration for 8 hours workday and 5 days workweek.
STEL:
Short Term Exposure Limit: 15 minute time weighted average which should not exceed any time during a workday.
V. Effects on Land and Water Bodies:
Particulate matter emitted from industrial units settle on land, buildings and other structures which give them a grimy look. Such deposits on land may interfere with soil fertility. Acid gases, such as CO2, NOx, SO2 react in the atmosphere and are deposited on land and water bodies either in the form of acid rain by combining with rain water or as dry salt particles.
Such acid deposition on land would adversely affect land fertility and vegetation growth. Plant nutrients and micro-nutrients present in soil would be leached away by acid rain as a result of which the affected land would not be able to support growth of various types of plants. Because of poor vegetation cover, rain water and wind will carry away the top soil layer and finally may turn the area arid.
Acid deposition on water bodies in general would affect the growth of aquatic plants and fish. However, fresh water bodies, which constitute only a very small fraction of the total hydrosphere, would be seriously affected by acid rain and salt deposits. Industrial units and human settlements located near the fresh water bodies would get affected once the water bodies would get affected due to acid rain.
VI. Effects on Materials:
Air-borne gaseous pollutants and particles affect buildings and other structures, equipment and machinery. Gaseous pollutants may directly attack building materials, metals and non-metals by reacting irreversibly. In the case of metals the mechanism of attack may be electro chemical in nature.
ADVERTISEMENTS:
The extent of damage would depend on the temperature of the environment and the presence of moisture. Even in those cases where the surfaces are not directly attacked chemically but are covered by particle, they get damaged during the process of cleaning. The surfaces also get corroded by acid rain.
Particulate matter damages surfaces of equipment and machinery by abrasion and by getting lodged in between the moving parts. Particulate matter and gaseous pollutants may affect sensitive components of instruments and equipment either by getting deposited or by reacting chemically.
B. Stratospheric Effects:
In the stratosphere very little emission of pollutants occurs directly. The only direct sources of emission are supersonic air-crafts and rockets. The exhaust from such sources may contain CO, CO2 NOx, SO2, H2O and hydrocarbons depending on the fuel used.
Some pollutants also reach the stratosphere directly due to volcanic eruption. Other than these, some gaseous pollutants, which escape, complete chemical transformation in the troposphere, diffuse into the stratosphere. These are mainly NOx, chlorofluorocarbons and some hydrocarbons.
The above-mentioned pollutants react with the ozone molecules present in the ozone layer (of the stratosphere) and undergo oxidation. Absorption of UV solar radiation by the ozone molecules and the oxidation reactions (of Pollutants) cause a little warming of the stratosphere. This results in a slight positive (vertical) temperature gradient in the stratosphere.
In the ozone layer ozone molecules are produced by photochemical reactions as a result of absorption of solar UV radiation. Some of the ozone molecules get destroyed due to reactions with the pollutants present. As the concentration of the pollutants is increasing in the stratosphere the rate of destruction of ozone molecules is becoming more than its rate of production. This process is causing depletion of ozone molecules in the ozone layer.
The pollutants, which have been identified as the major agents responsible for depletion of ozone, are the chlorofluorocarbons (CFC). CFC is used as refrigerants in refrigerators and air-conditioners. Those are also used as aerosol spray, solvents, cleaning agents, foam-blowing agents, etc. The other ozone depleting substances (ODS) are methyl chloroform, carbon tetrachloride, methyl bromide and halons.
The CFC molecules, which find their way to the atmosphere, do not react chemically with oxygen in the troposphere but diffuse to the stratosphere where UV radiation dissociates them and produces chlorine atoms. The atoms act as a catalyst in the conversion of ozone into oxygen. As a result of this process the concentration of ozone molecules in the ozone layer is getting depleted.
The ozone layer plays a very important role in protecting living beings on the earth by absorbing high-energy solar UV radiation (220-330 nm). Progressive depletion of the ozone layer would result in more infiltration of UV radiation, which would cause more harm to the living beings including man.
Human health gets affected as a result of overexposure to UV radiation as it causes skin cancer, cataracts and immune system suppression. UV radiation also damages plants, marine ecosystem and synthetic as well as natural polymers.
Scientists, politicians and even common people, over the world, are concerned about the progressive depletion of the ozone layer. At some places (for example, at the South Pole) the ozone layer has become very thin, which is referred to as ‘ozone hole’.
To forestall further reduction of the ozone layer and also to enhance its regeneration an International Agreement (Montreal Protocol) was signed in 1987 at Montreal. It was agreed that CFC production would be halved by 1995 and completely phased out by 2000. The Protocol was substantially amended in 1990 and 1992.
The developed countries agreed to phase out CFC with HCFC (hydro chlorofluorocarbon) by 2000. HCFC mostly undergoes chemical transformation in the troposphere and the lower stratosphere and hence would not affect the ozone layer too much.
It has been envisaged to phase out HCFC with HFC (hydro fluorocarbon) which is more ozone-friendly having zero ozone depletion potential. The United Nation Environmental Programme (UNEP) has proposed that HCFC production should be stopped by 2005.