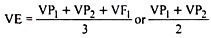ADVERTISEMENTS:
Isotopes are chemical elements that have the same atomic number (i.e., the number of protons in the nucleus of the atom) but different atomic masses (i.e., the sum of the number of protons and neutrons in the nucleus).
Certain isotopes are unstable and undergo spontaneous nuclear changes (called transmutations) accompanied by the emission of particulate and sometimes also electromagnetic radiations.
These atoms are said to be radioactive and are called radioisotopes or radionuclides. Radioactive atoms may readily be detected by instruments sensitive to their radiations.
ADVERTISEMENTS:
Generally, an organism cannot distinguish between the stable and radioactive forms of the same element so that both are metabolized in an identical manner.
It is for this reason that radioisotopes have proven extremely useful to biologists, as these elements may be conveniently employed as tracers. That is, the fate of a given element (or molecule) in an organism or individual cell may be studied by introducing the radioactive form of that element and following the uptake and subsequent localization of the radioactivity. Some of the radioisotopes frequently employed as tracers are listed in Table 14-1.
If the radioisotope is initially a part of a molecule, then the fate of all or part of that molecule may similarly be followed. The use of radioisotopically labeled compounds is particularly desirable when the compound to be administered is a normal constituent of the cell or organism and would be impossible to distinguish from stable molecules already present.
ADVERTISEMENTS:
Many organic and inorganic compounds of biological interest may now be obtained that have one or more specific atomic positions occupied by radioisotopes. Because of the extremely high sensitivities of many radiation detectors, the quantities of radioisotopes employed in tracer studies can be kept small enough to preclude significant damage to cell constituents by the radiation.
Advantages of the Radioisotope Technique:
Results obtained from experiments in which radioisotopes are used are quantitative, because the amount of radioactivity present and available for detection is directly proportional to the radioisotope content of the sample being analyzed. Moreover, numerous biological studies carried out on a routine basis with radioisotopes can only be performed with great difficulty or are virtually impossible without them. Some examples are cited to illustrate the value of the radioisotope technique.
Determination of Molecular Fluxes under Conditions of Zero Net Exchange:
The movement of a particular ionic or molecular species between different tissues of an organism or between a cell and its surroundings is often in dynamic equilibrium, that is, although a continuous exchange of a given substance between one region and another takes place, no net transfer of material occurs.
Alternatively, the concentration of a given substance within a tissue or cell-may remain fairly constant as a result of the balanced biosynthesis and degradation of that material. These situations cannot be easily detected or studied by routine chemical analyses. The continuous flux of Na+ and K+ across the plasma membrane of the erythrocyte is a typical example of this type of equilibrium.
The concentration of K + within the mammalian erythrocyte is much higher than in the surrounding blood plasma, whereas the reverse is true for Na+. These large concentration differences are sustained in spite of the continuous passage of Na + and K + across the plasma membrane in both directions. Consequently, this dynamic steady state is not revealed by chemical analysis and, prior to the utilization of radioisotopes of sodium and potassium to study this situation, the concentration differences were interpreted as the consequence of the impermeability of the erythrocyte’s plasma membrane to Na+ and K + .
When the radioisotopes-of sodium and potassium, 24Na and 42K, were used to label the plasma surrounding the red cells, the passage of radioactivity into the erythrocytes was observed, eventually approaching equilibrium identical to the concentration equilibrium determined chemically. These studies unequivocally demonstrated that the plasma membrane of the red cell is readily permeable to Na+ and K+ and that a continuous exchange of these two ions between the cell and the surrounding plasma takes place.
Observations of this kind provided great impetus to the development of concepts concerning the continuous metabolic turnover of cellular materials present in constant concentrations and the active transport of materials across the plasma membrane against concentration gradients.
ADVERTISEMENTS:
In addition to providing qualitative information, radioisotopes may be used to accurately measure the rates at which the metabolic turnover of materials within a cell or tissue occurs, as well as the rates of exchange of materials across cellular membranes. Prior to the availability of radioisotopes, no satisfactory methods were available to measure these rates. In the case of the erythrocyte, it has been shown that about 2% of the cell’s Na+ and K+ are exchanged with the plasma each hour.
Simplification of Chemical Analyses:
Depending on the nature of the chemical analyses required quantitative determinations of the distribution of a given element or compound in different tissues of the body or in different parts of an individual cell may be very difficult. This difficulty may be compounded if certain components to be analyzed contain only trace amounts of the substance to be measured. Because radioisotope-containing compounds are not distinguished from their stable (nonradioactive) counterparts by an organism, the use of labeled compounds in studies of this sort can greatly simplify and reduce the number of analyses.
Two requirements must be fulfilled:
ADVERTISEMENTS:
(1) The administration of the labeled compound must quickly be followed by its uniform distribution among nonradioactive molecules of the same kind that are already present in the system and
(2) Once a uniform distribution is attained, the specific activity of the substance in question must be determined in one of the several components (i.e., tissues, cell fractions, etc.) to be analyzed. Specific activity is defined here as the quantity of radioactive element or compound per unit mass of total element or compound (the units of measurement are described later).
If the labeled compound does become uniformly undistributed in the system, then the specific activity will be equal throughout. Consequently, all subsequent measurements of the quantity of that compound may be made simply by measuring the amount of radioactivity present in the tissue or cell part to be analyzed. In nearly every case, this is far easier than performing a series of quantitative chemical measurements of the element or compound in question.
The rates at which different elements or compounds are incorporated by individual cells or by the body tissues, together with a determination of the specific loci of the deposition, can also be conveniently determined using radioisotopes.
ADVERTISEMENTS:
For example, cells may be incubated in a medium containing labeled material or, in the case of measurements in whole animals the material may be introduced into the bloodstream. In these cases, the specific activity of the element or compound is determined before it is made available for incorporation and the subsequent rate of incorporation is determined either from the rate of disappearance of radioactivity from the medium or bloodstream or from the rate of appearance of radioactivity in the cells or tissue.
The specific locus of deposition or utilization within the cell may be determined from separate radioactivity measurements of fractionated tissue or by auto radiographic procedures (to be described later in this chapter) combined with light or electron microscopy. Again, it should be noted that specific quantitative chemical analyses of the material being incorporated by the .cells are unnecessary when the radioisotope technique is applied.
“Isotope Dilution” Methods:
A common problem confronting physiologists and one’ that especially lends itself to the radioisotope technique is the determination of the total quantity or volume of a given material in the body or in a cell when quantitative isolation of that material for analysis is not possible.
ADVERTISEMENTS:
Determinations of total circulating blood volume, erythrocyte mass, chloride space, exchangeable sodium, and so on fall in this category. The manner in which such unknown quantities are measured using radioisotopes may be exemplified by considering the following generalized case. Suppose that material X occurs in a system (i.e., a tissue, cell, etc.) in unknown abundance and a labeled form of this material, X*, is either available commercially or can be prepared experimentally with a known specific activity, SA, given in this instance by the equation
SA = (X*)/ (X*+X) (14-1)
A measured quantity of the labeled material is introduced into the system and permitted to equilibrate thoroughly with the nonradioactive material that was already present. Following this, a small quantity of the material is withdrawn from the system and its specific activity is determined.
Because the X* originally introduced was uniformly mixed with unlabeled X already present, its specific activity will have been reduced in direct proportion to the total amount of X present in the system. Consequently, the total amount of X originally present can be determined from the relationship
XT = X* (SA1+SA2 – 1) (14-2)
Where
ADVERTISEMENTS:
XT=the total amount of X originally present in the system,
X* = the quantity of labeled material added,
SA1 = the original specific activity of the material added, and
SA2 = the specific activity of the sample removed for analysis.
The isotope dilution technique is a fundamental part of radioimmunoassay procedures.
Properties of Radioactive Isotopes:
ADVERTISEMENTS:
Most radiations emitted by radioisotopes are the result of changes in the unstable atomic nuclei. Whether a given atomic nucleus is stable-depends in turn on the numbers of neutrons (N) and protons (Z) that it contains. The relationship between nuclear stability and the neutron: proton composition of the nucleus is shown in Figure 14-2.
All stable (and a number of unstable) isotopes have N and Z values that fall within the shaded zone. Note that for the lighter elements, nuclear stability exists when N=Z, whereas in the stable, heavier elements, the number of neutrons exceeds the number of protons, with the allowable neutron excess increasing with atomic number.
Isotopes with N and Z numbers outside of the shaded zone (as well as some that occur within the zone) shown in Figure 14-2 undergo spontaneous changes in which neutrons and protons are inter converted. The resulting change establishes a stable combination of N and Z values. These intra-nuclear changes are accompanied by the emission of particulate and sometimes electromagnetic radiation.
Types of Radiation Emitted by Radioisotopes:
The most common types of nuclear radiations are alpha particles, positive and negative beta particles, and gamma rays. Alpha particles, which consist of two protons and two neutrons and are therefore identical to helium nuclei, are emitted by radioisotopes of high atomic number (e.g., uranium, polonium, thorium, and radium) and are rarely used as tracers in biological studies.
Positive beta particles, also called positrons, are emitted from nuclei in which the N:Z ratio is lower than that which is stable. The nuclear transmutation involves the conversion of a proton into a neutron, positron, and neutrino, the latter two being ejected from the nucleus. The positron possesses a unit positive charge and is equal in mass to an electron, whereas the neutrino has neither mass nor charge. The isotope 116C decays by positron emission (the numbers that precede the element’s symbol are its atomic mass [the superscript] and its atomic number [the subscript]):
In view of the similarity between beta particles and electrons, the symbol “e” is often used to describe the beta particle. Very few positron-emitting radioisotopes are employed as biological tracers. Nearly all radioisotopes used as tracers by biologists emit negative beta particles (negatrons). It has become customary to drop the term “negative” so that the expression “beta particle” is understood to imply negative beta particle.
Although technically incorrect, this terminology is widely used and will be employed here. Beta particles are emitted from nuclei in which the N:Z ratio is greater than that which is stable. The nuclear change involves the conversion of a neutron to a proton with the resulting ejection of a beta particle and neutrino. 116C and 3212P may serve as examples of this form of decay:
The emission of beta particles from the nuclei of 3H, 14C, 32P, ass, sea, and 45Ca atoms changes the N:Z ratio to a stable value and lowers the energy content of the nucleus to the ground state. The energy lost by the nucleus in this process is divided between the beta particle and neutrino.
However, for many radioisotopes (e.g., 24Na, 59Fe, and 131I), intra nuclear changes resulting in the emission of beta particles and neutrinos produce nuclei with stable N:Z ratios but with energy levels that are still above the ground state. In these instances, the excess energy is eliminated and the ground state is attained by the emission of one or more gamma rays; for example,
Unlike alpha and beta radiation, gamma radiation is not particulate but is electromagnetic.
Energy of Radiation and Its Interaction with Matter:
Radiation energy is measured in electron volts (abbreviated eV), one electron volt being the kinetic energy acquired by an electron in an electrical potential difference of one volt. The beta particles and gamma rays emitted by radioisotopes that are often used as tracers have energies ranging from about 1 x 104 to 4 x 106eV or 0.01 to 4.0 MeV (1 MeV equals 1 million electron volts).
This energy range may be compared with the energy of chemical bonds, which is on the order of a few electron volts. The kinetic energy acquired by a beta particle during transmutation of the parent atom’s nucleus can vary from 0 MeV up to some maximum value, Emax, which is a characteristic of the particular radioisotope. As an example, the energy spectrum for 32P beta particles is shown in Figure 14-3.
It should be noted that the maximum energy of a 32P beta particle is 1.71 MeV but that the average energy is much less (0.70 MeV). Although the value for Emax varies, the shape of the energy curve is similar for all beta particle-emitting radioisotopes.
To reduce the energy level of the radioisotope nucleus to the ground state, a specific quantity of energy, Q, must be given off as radiation during the nuclear change. For isotopes emitting only beta particles, Q equals Emax and the energy of the neutrino accounts for the difference between Emax and the actual kinetic energy acquired by the beta particle.
For example, if the transmutation of a particular 32P atom results in the emission of a 1.20-MeV beta particle, then the accompanying neutrino would have an energy of 0.51 MeV (i.e., 1.20 MeV + 0.51 MeV = 1.71 MeV = Emax). If the beta particle has escaped the nucleus with the maximum possible energy, then no neutrino would have been emitted. Table 14-2 lists the maximum beta particle energies of some radioisotopes.
In contrast to beta particles, gamma rays are emitted at specific energy levels. Thus, all gamma rays emitted by 42K have an energy of 1.5 MeV (Table 14- 2). The Q value of an isotope emitting both beta and gamma radiation is equal to the sum of the energies possessed by the beta particle, neutrino, and subsequent gamma ray(s).
Occasionally, as in the case of 24Na, the emission of a beta particle may be followed by the emission of two (or more) successive gamma rays in order to reduce the nuclear energy level to the ground state. It may also be noted from Table 14-2 that the decay of some radioactive isotopes (e.g., 42K, 59Fe, and 131I) proceeds along two or more alternative pathways, that is, beta particles having more than one Emax may be emitted, and because the Q value is constant, these are necessarily followed by gamma rays of different energies.
Beta particles and gamma rays interact with matter by ionizing and exciting atoms in their path. In the case of beta particles, ionization results from the repulsion of orbital electrons by the negatively charged particle. As a result beta particles tracking through matter produce a wake of electrons and positively charged ions (called ion pairs).
The number of ion pairs produced per unit path length, called the specific ionization, is not constant but increases as the beta particle slows down. Also, the path of the beta particle is not linear but is quite erratic as a result of its repulsion by the orbital, electrons of atoms with which it interacts.
High-energy beta particles may traverse the linear equivalent of 1 to 2 m in air. Because gamma radiation is electromagnetic, its probability of interacting with matter is less than that for beta particles. Consequently, gamma rays have a much lower specific ionization and a much longer and also linear path length.
Half-life:
The number of atoms in a sample of radioisotope that disintegrate during a given time interval decreases logarithmically with time and is unaffected by chemical and physical factors that normally alter the rates of chemical processes (i.e., temperature, concentration, pressure, etc.). Radioactive decay is therefore a classic example of a first-order reaction. A convenient term used to describe the rate of decay of a radioisotope is the physical half-life, Tp. This is the amount of time required to reduce the amount of radioactive material to one-half its previous value.
Each radioactive isotope decays at a characteristic rate and therefore has a specific half-life (see Table 14-1). For example, the amount of radioactivity arising from a sample of 59Fe is reduced to one-half its original value in 45.1 days, to one-fourth in 90.2 days, to one-eighth in 135.3 days, and so on. The amount of decay occurring in the course of a tracer experiment must be taken into account when radioisotopes of short physical half-life such as 24Na, 32P, 42K, 59Fe, 131I, and 125I are used. Of course, this is not a problem in tracer experiments employing 3H and 14C (Table 14-1) because the length of the experiment is insignificant in comparison with the half-life.
When radioisotopes are used in vivo experiments of extended duration, the turnover rate of the element in the body (or in the cell) must also be considered, for the rate of decrease of radioactivity will be a function in both radioactive decay and metabolic turnover. In these instances, a more useful term is the effective half-life, Te, which is the amount of time required to reduce the radioisotope content of the body (or cell) to one-half its original value by the combined effects of decay and turnover; it is determined using the relationship
Where
Te = effective half-life,
Tp = physical half-life, and
Tb = biological half-life and is defined as the normal amount of time required for the turnover of one-half of the body content of a given element (radioactive or nonradioactive).
The physical, biological, and effective half-lives of several elements are compared in Table 14-3. It should be noted that Te can never be greater than Tp and that the slower the rate of turnover of an element, the closer Te approaches Tp.
Detection and Measurement of Radiation:
The selection of instruments for the detection and measurement of radioisotopes is based primarily on the type and energy of the emitted radiation. The most commonly used detectors are: (1) Geiger- Muller counters, which are employed primarily with isotopes emitting beta particles of intermediate or high energy (Emax above 0.2 MeV) and which may also be used at low efficiency for the measurement of gamma radiation; (2) solid scintillation counters, which are generally employed with gamma ray- emitting isotopes; and (3) liquid scintillation counters, which are used with isotopes emitting low- energy beta particles (Emax below 0.2 MeV).
Geiger-Muller Counters:
The most widely used instrument for the detection and measurement of radiation is the Geiger-Muller (or G-M) counter. The detector itself, called a G-M tube, consists of a cylinder several inches long containing two electrodes and filled with a readily ionizable inert gas such as helium or argon.
The insulated metallic internal surface of the cylinder serves as the cathode and a narrow wire passing down the center of the tube serves as the anode (Fig. 14-4). One end of the G-M tube is covered by a thin material such as mylar plastic or mica and is called the end-window. The anode and cathode terminals at the other end of the tube are connected to a source of high voltage and a scaler, a device that simply counts electrical pulses.
When a radioactive sample (usually deposited on a small metal disk called a planchet) is placed near the end-window, radiation enters the G-M tube, ionizing some of the gas molecules and forming a number of ion pairs (i.e., positively charged argon or helium atoms and electrons). If a sufficiently high electrical potential is applied to the electrodes, the ion pairs will migrate toward the appropriate electrode. During this migration, the ions collide with and ionize additional gas molecules, so that the passage of a single beta particle through the gas results in a large number of ions being collected at the electrodes.
These events produce an electrical pulse that is recorded by the scaler as a count. Ideally, each ionizing ray entering the G-M tube is registered as a count and the amount of radioactivity is expressed as counts per minute (cpm). Because radiation is emitted in all directions from a radioactive source, it is apparent that only a small percentage of the rays arising from the sample are directed toward the end-window. Therefore, even if all the rays entering the G-M tube are detected and counted, the cpm recorded for the sample is only a fraction of the true rate of disintegration (i.e., disintegrations per minute, dpm) of the isotope.
This does not pose a serious problem when the relative isotope contents of a number of samples are to be determined (this is generally the case) and if constant geometric conditions are maintained for each sample (i.e., distance of the sample from the end-window, volume of sample, etc.). For some radioisotopes such as 3H, 14C; 35S, and others of low Emax, much or all of the energy of the emitted beta particles may be dissipated before the ray enters the ionizing gas. For example, the energy may be expended within the sample itself (called self- absorption), in the air between the sample and the end-window, or in the material of the end-window.
Even with radioisotopes emitting beta particles of high Emax (Table 14-2), beta particles in the low region of the energy spectrum may go undetected. Because of the low specific ionization of gamma rays and the low density of the gas in the G-M tube, gamma rays may pass through the tube without causing ionizations and therefore go undetected. For these reasons, Geiger-Muller counters are usually not suitable for the detection and measurement of radioisotopes emitting gamma rays or beta particles of low Emax.
Even when no radioactive sample is placed below the end-window of the G-M tube, a small count is recorded. This is known as the background count and results from cosmic radiation (primarily gamma rays), naturally occurring radioisotopes in laboratory materials (such as 40K in glass and naturally occurring 14C and 3H in organic compounds), radioactive samples left in the vicinity of the detector and electronic “noise” within the components of the counting system.
Therefore, the background count must always be subtracted from the count obtained for a radioactive sample. The magnitude of the background count may be reduced by placing lead shielding around the detector so that much of the cosmic radiation and radiations from other sources are absorbed before reaching the detector.
The total amount of radioisotope present in a sample at any instant in time may be determined from its rate of disintegration, that is, its dpm; the basic unit of measurement is the curie (Ci) and is defined as that quantity of radioisotope undergoing 2.22 x 1012dpm. (Note that the curie content of a radioactive sample decreases exponentially with time at a rate determined by the physical half-life of the radioisotope.)
In most tracer experiments, the quantity of radioisotope used is generally at the millicurie (mCi) or microcurie (μCi) level. The curie content of a labeled compound is generally provided at the time of purchase so that the efficiency of the counting system may be determined by comparing the recorded cpm of an aliquot of the isotope with its known dpm.
Generally, this value is 10% or less for G-M counters but is much higher in solid and liquid scintillation systems. Once the efficiency of the counting system is known, then the specific activity of a radioactive sample (which we may now define as the number of curies per unit mass of element or compound) collected during the course of a tracer experiment may be calculated from its observed counting rate and composition. G-M counters are effectively employed in tracer experiments involving 24Na, 32P, 36C1, and other “hard beta” emitters but generally are not used with 3H and 14C, which emit “soft beta” rays.
Solid Scintillation Counters:
Solid scintillation counters are used to detect and measure radioisotopes emitting gamma rays. The detector (Fig. 14-5) consists of a large crystal of thallium-activated sodium iodide and a photomultiplier tube encased in aluminum housing; the latter is interfaced with a preamplifier, a source of high voltage, and a scaler.
The radioactive sample to be analyzed is placed either against the end of the detector containing the crystal or, for greatly improved counting efficiency, into a well-shaped opening drilled into the crystal’s surface (Fig. 14-5).
Because of its high density, the crystal absorbs much of the energy of the gamma rays, causing excitation of electrons of atoms composing the crystal and raising them to higher energy orbitals. As these electrons return again to their lower energy orbitals, flashes of light or scintillations are emitted and these are proportional in number to the number and energy of the gamma rays exciting the crystal.
The light photons are converted by the photomultiplier tube into electrical pulses of corresponding magnitude and frequency and these are relayed to the scaler. Because the magnitude of the electrical pulses produced is proportional to the energy of the gamma rays, and because gamma rays are mono- energetic, the inclusion of the appropriate circuitry in the counting system (i.e., a pulse-height analyzer) allows different gamma ray-emitting isotopes to be distinguished.
In contrast to G-M counters, few or no problems involving self-absorption and end-window absorption are incurred when solid scintillation methods are used with gamma ray-emitting isotopes. However, the use of constant geometry and lead shielding around the detector to reduce the magnitude of the background count is important.
Radioimmunoassay:
At the present time, one of the most common uses of solid scintillation counting is in certain radioimmunoassay (RIA) procedures. The RIA technique, developed in the 1950s by S. A. Berson and R. S. Yalow, is a tracer procedure used to measure trace amounts of any substance with antigenic properties.
Depending on the specific activity of the labeled compound, quantities of antigen that are as low as 10 pictograms (i.e., 10 millionths of a microgram) may be detected. Prior to the development of RIA procedures, the assay of biological substances that did not possess enzymatic activity or some other easily measurable property was extremely difficult.
However, because many different kinds of chemical substances are antigenic (i.e., they can elicit an antibody response), they can be reliably quantitated using the RIA procedure. The sensitivity of the RIA technique equals or surpasses all chromatographic and spectrophotometric methods currently in use. The technique has been especially useful for assaying hormones (Table 14-4).
The RIA procedure is depicted schematically in Figure 14-6. A sample containing the antigen to be quantified is mixed with a second sample containing radioisotopically labeled antigen of very high specific activity. To the mixture is added antiserum containing antibody previously prepared against the antigen.
The amount of antibody added must be limiting so that antigen is always in excess (even when no unlabeled antigen is present). The resulting antigen-antibody reaction produces a complex in which the amount of radioisotope present is inversely proportional to the original quantity of unlabeled antigen in the sample being assayed. In the final stage of the radioimmunoassay, the antigen-antibody complex is separated from free antigen by precipitation.
As illustrated in Figure 14-6, the antiserum contains enough antibody to bind 12 antigen molecules. If 12 labeled and 12 unlabeled antigen molecules are present, then only one-half of the labeled antigens can be bound. If 24 unlabeled antigen molecules are present (as in the diagram), then only one-third of the labeled antigens can be bound, and so on. Thus, the radioisotope content of the isolated antigen-antibody complexes can be used as a measure of the amount of unlabeled antigen present in the original sample.
When the antigen is all or part protein or peptide, the labeled form is prepared by iodination with either 131I or 125I. The iodine is incorporated into the benzene ring of tyrosine residues. Both of these radioactive tracers emit gamma rays. In radioimmunoassay, radioactive tracers of iodine are preferred to those of hydrogen and carbon because 557 atoms of 3H and 261,672 atoms of 14C must be incorporated into each antigen molecule to yield the same dpm as one atom of 131I or seven atoms of 125I. Although I31I emits more energetic and more easily detected gamma rays, 125I is usually the isotope of choice because of its longer half-life (60 days versus 8.1 days).
Liquid Scintillation Counters:
Except perhaps in the case of the radioimmunoassay of proteins, 3H and 14C are used more extensively in tracer experiments than any other radioisotope. This is not surprising in view of the ubiquitous occurrence of hydrogen- and carbon-containing compounds in cells.
The energies of the beta particles emitted by these isotopes are very low (especially tritium), so that it is only with great difficulty that they can be accurately measured using gas ionization methods. The great demand for a sensitive and accurate method for measuring 3H and 14C, as well as other soft beta emitters, led in the 1950s to the development of the liquid scintillation technique.
Before considering this important technique in detail, the basic mechanism involved in the detection process will be briefly summarized. The radioactive sample to be counted is mixed in a glass or plastic vial with a “scintillation fluid.”
Certain molecules dissolved in the fluid indirectly absorb the energy dissipated by the beta particles and respond by scintillating. The resulting photons of light are detected by photomultiplier tubes that relay proportional electrical pulses to the scaling units.
Certain advantages of the procedure are immediately apparent, for if the sample is thoroughly mixed with the scintillation fluid, no self-absorption occurs. Moreover, because the technique is employed almost exclusively with weak beta-emitting isotopes, the energy of all beta rays is dissipated within the fluid, making it possible, at least in theory, to detect most if not all of the nuclear transmutations.
The scintillation fluid contains three major components:
(1) An organic solvent constituting the bulk of the mass,
(2) A primary solute (fluor), and
(3) A secondary solute (fluor).
The most commonly used solvents and fluors are listed in Table 14-5. The energy of the beta particle is transferred to the solvent, causing ionization or excitation of solvent molecules.
The latter effect is more important, because in returning to the nonexcited ground state, energy is transferred to the primary fluor molecules, causing their excitation. In returning to the ground state, the excited primary fluor emits photons of near ultraviolet light.
The photocathodes of most photomultiplier tubes are not sensitive to photons having wavelengths in this region, and it is the role of the secondary fluor to absorb the light energy from the primary solute and reemit it as light of longer wavelength. (Secondary fluors are sometimes called “wave shifters.”) For example, the light emitted by PPO has a wavelength maximum at 365 nm, whereas POPOP emits light having a wavelength maximum at 420 nm, which is close to the maximum sensitivity region of most photomultiplier tubes.
The size of the electrical pulse produced by the photomultiplier tube is proportional to the energy dissipated by the beta particle in the liquid scintillation fluid. Consequently, a pulse-height spectrum is produced having a profile almost identical to that of the beta particle energy spectrum. In the case of 3H and 14C, the sizes of many of the pulses produced are so low that spurious low-amplitude pulses arising spontaneously within the photomultiplier tube itself (i.e., “noise”) result in a falsely high count.
To minimize this problem, in most liquid scintillation counters the sample vial is placed between the faces of two photo- multiplier tubes connected through a pulse-height analyzer to coincidence circuitry (Fig. 14-7) that permits a count to be registered only when simultaneous pulses are produced by the tubes.
Therefore, a noise signal from one photomultiplier tube is not recorded unless another noise signal is produced by the other tube at the same time (actually, within 10-7 seconds of each other). Because noise signals are random, coincidental noise pulses are few in number. As in G-M and solid scintillation counting, the background count must be subtracted from each recorded count. In liquid scintillation counting, the background count is derived from a number of sources including coincidental tube noise, naturally occurring radioisotopes in the vial and scintillation fluid (most glass contains small amounts of 40K and organic solvents contain small amounts of 3H and 14C, and cosmic radiation.
Most liquid scintillation counters contain pulse- height analyzers that provide for the rejection of pulses above and below a selected size range. This feature is particularly valuable in “double-label”, experiments (experiments in which two different radioisotopes are used). Because the spectrum of pulse heights produced is related to the beta particle energy spectrum of the radioisotope, the settings of the pulse- height analyzer may be adjusted to distinguish pulses arising from each of the isotopes present in the sample. This is particularly easy in the case of samples that contain both 3H and 14C, as their beta particle energy spectra differ so markedly (Fig. 14-8).