ADVERTISEMENTS:
This article throws light upon the microbial production of 7 types of amino acids. The seven types are: (1) Amino Acid (2) L-Glutamic Acid (3) L-Lysine (4) L-Threonine (5) L-Phenylalanine (6) L-Tryptophan and (7) L-Aspartic Acid.
Type # 1. Amino Acid:
Some general considerations on the production methods, and the development of strains of microorganisms for improved amino acid production are briefly described.
Methods for Production of Amino Acids:
The industrial production of amino acids is carried out by one or more of the following three processes:
ADVERTISEMENTS:
1. Extraction:
Amino acids are the building blocks in protein structure. The proteins can be subjected to hydrolysis, and the requisite amino acids can be isolated e.g. cysteine, tyrosine, leucine.
2. Chemical synthesis:
Chemical synthesis results in a mixture of D- and L-amino acids. Most of the amino acids required for commercial applications are of L-category. However, for the synthesis of glycine (optically inactive) and some other amino acids which can be used in L- or D-form (D, L-alanine, D, L-methionine) for certain purposes, chemical methods are employed.
ADVERTISEMENTS:
3. Microbiological production:
For the large- scale production of amino acids, microbiological methods are employed. There are three different approaches.
(a) Direct fermentation methods:
Amino acids can be produced by microorganisms by utilizing several carbon sources e.g. glucose, fructose, alkanes, ethanol, glycerol, propionate. Certain industrial byproducts like molasses and starch hydrolysate can also be used. Methanol, being a cheap carbon source, is tried for amino acid production, but with limited success.
(b) Conversion of metabolic intermediates into amino acids:
In this approach, the microorganisms are used to carry out selected reactions for amino acid production e.g. conversion of glycine to serine.
(c) Direct use of microbial enzymes or immobilized cells:
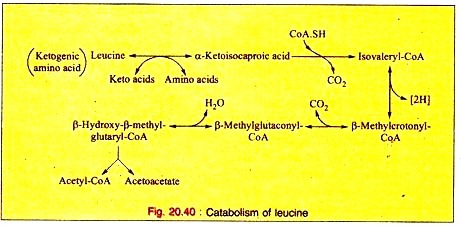
Sometimes resting cells, immobilized cells, crude cell extracts or enzyme-membrane reactors can be used for the production of amino acids. Some examples are given below. Amino acid dehydrogenases from certain bacteria (e.g. Bacillus megaterium) can be used for the amination of α-keto acids to produce L-amino acids e.g. alanine (from pyruvate), leucine (from α-ketoisocaproic acid) and phenylalanine (from phenyl pyruvate). Immobilized cells or enzyme- membrane reactors can be used. Enzymes or immobilised cells are also employed for the production of several other amino acids e.g. tryptophan, tyrosine, lysine, valine.
Strain Development for Amino Acid Production:
The metabolic pathways, for the synthesis of amino acids by microorganisms, are tightly controlled and they operate in an economical way. Therefore, a natural overproduction of amino acids is a rare occurrence. Some strains that excrete certain amino acids have been isolated e.g. glutamic acid, alanine, valine.
ADVERTISEMENTS:
In order to achieve an overproduction of any amino acid by a microorganism, methods have to be devised for the elimination of the metabolic regulatory/control processes. In fact, several amino acid-producing microorganisms have been developed by mutagenesis and screening programmes.
The following are the major ways of strain development. In fact, several methods are combined to successfully develop a new strain for producing amino acids.
Auxotrophic mutation:
These mutants are characterized by a lack of the formation of regulatory end product (i.e. repressor or regulatory effector). The intermediates of the metabolic pathways accumulate and get excreted.
ADVERTISEMENTS:
Genetic recombination:
Mutants can be developed by genetic recombination for overproduction of amino acids. Protoplast fusion in certain bacteria is used for development of hybrids e.g. Corynebacterium glutamicum and Bacillus flavum.
Recombinant DNA technology:
The classical techniques of genetic engineering can be used for strain development. Strains with increasing activities of rate-limiting enzymes have been developed. In one of the techniques, E: coli and cloning vector pBR322 were used to increase the genes for the production of amino acids e.g. glutamic acid, lysine, phenylalanine, valine.
ADVERTISEMENTS:
Functional genomics: a new approach:
Analysis of genomes from the wild and mutant strains of microorganisms will help in creating improved strains. Once the entire sequence of the chromosomes in the organisms (e.g. C. glutamicum, E. coli) is established, efforts can be made to carry out genetic manipulations for efficient overproduction of desired amino acids. Chip technology can be used to detect new mutations and consequently the fermentation processes.
Type # 2. L-Glutamic Acid:
L-Glutamic acid was the first amino acid to be produced by microorganisms. The original bacterium, Corynebacterium glutamicum, that was first used for large scale manufacture of glutamic acid continues to be successfully used even today. The other important organisms (although used to a lesser extent due to low yield) employed for glutamic acid production belong to genera Micro bacterium, Brevibacterium and Arthrobacter.
All these organisms have certain morphological and physiological characters comparable to C. glutamicum. Biochemically, glutamic acid- producing bacteria have a high activity of glutamate dehydrogenase and a low activity of α-ketoglutarate dehydrogenase. They also require the vitamin biotin.
Improved Production Strains:
ADVERTISEMENTS:
Several improvements have been made, particularly in C. glutamicum, for improving the strains to produce and excrete more and more of glutamic acid. These include the strains that can tolerate high concentrations of biotin, and lysozyme-sensitive mutants with high yield.
Biosynthesis of L-glutamic Acid:
The pathway for the synthesis of glutamic acid with glucose as the carbon source is depicted in Fig. 26.1. Glucose is broken down to phosphoenol pyruvate and then to pyruvate. Pyruvate is converted to acetyl CoA. Phosphoenol pyruvate (by the enzyme phosphoenol pyruvate carboxylase) can be independently converted to oxaloacetate. Both these carboxylation reactions are quite critical, and require biotin as the cofactor.
The next series of reactions that follow are the familiar citric acid (Krebs) cycle reactions wherein the key metabolite namely α-ketoglutarate is produced. In the routine citric acid cycle, α – ketoglutarate is acted upon by the enzyme α- ketoglutarate dehydrogenase to form succinyl CoA.
For the production of glutamic acid, α-ketoglutarate is converted to L-glutamic acid by the enzyme glutamate dehydrogenase (GDH). This enzyme is a multimer, each subunit with a molecular weight of 49,000. The reducing equivalents, in the form of NADPH + H+, are required by GDH. They are generated in the preceding reaction of Krebs cycle (catalysed by the enzyme isocitrate dehydrogenase) while converting isocitrate to α-ketoglutarate. The supply and utilization of NADPH + H+ occurs in a cyclic fashion through the participation of the two enzymes, namely isocitrate dehydrogenase and glutamate dehydrogenase (Fig. 26.2).
Theoretically, one molecule of glutamic acid can be formed from one molecule of glucose. In practice, the conversion efficiency of glucose to glutamic acid was found to be around 70%.
Regulation of Glutamic Acid Biosynthesis:
ADVERTISEMENTS:
The essential requirement for glutamic acid production is the high capability for the supply of the citric acid cycle metabolites. This is made possible by an efficient conversion of phosphoenol pyruvate as well as pyruvate to oxaloacetate .Thus, there are two enzymes (phosphoenol pyruvate carboxylase and pyruvate carboxylase) to efficiently produce oxaloacetate, while there is only one enzyme (pyruvate dehydrogenase) for the formation of acetyl CoA.
Certain microorganisms which have either phosphoenol pyruvate carboxylase (e.g., E. coli) or pyruvate carboxylase (e.g. B. subtilis) are not capable of producing glutamic acid to any significant extent. C. glutamicum has both the enzymes and therefore can replenish citric acid cycle intermediates (through oxaloacetate) while the synthesis of glutamic acid occurs.
Another key enzyme that can facilitate optimal production of glutamic acid is α-ketoglutarate dehydrogenase of citric acid cycle. Its activity has to be substantially low for good synthesis of glutamic acid, as is the case in C. glutamicum.
Further, exposing the cells to antibiotics (penicillin) and surfactants reduces the activity of α-ketoglutarate dehydrogenase while glutamate dehydrogenase activity remains unaltered. By this way, oxidation of α-ketoglutarate via citric acid cycle can be minimised, while the formation of glutamic acid is made maximum possible.
Release of Glutamic Acid:
Glutamic acid is synthesized intracellularly, and therefore its release or export is equally important. It now appears that there is a carrier-mediated energy-dependent active process involved for the export of glutamic acid.
ADVERTISEMENTS:
There are several ways of increasing the membrane permeability for exporting glutamic acid:
i. Biotin limitation
ii. Addition of saturated fatty acids
iii. Addition of penicillin
iv. Use of oleic acid auxotroph’s
v. Use of glycerol auxotroph’s
vi. Addition of local anesthetics
vii. Addition of surfactants (Tween 40).
The effect of biotin deficiency in facilitating the release of intracellular glutamic acid has been worked out. Biotin is an essential cofactor (required by the enzyme acetyl CoA carboxylase) for the biosynthesis of fatty acids. Due to a limited supply or deficiency of biotin, fatty acid biosynthesis and consequently phospholipid synthesis is drastically reduced. As a result, membrane formation (protein- phospholipid complex) is defective which alters permeability for an increased export of intracellular glutamic acid.
It is found that there is an alteration in the membrane composition of phospholipids in oleic acid and glycerol auxotroph mutants. This facilitates release of intracellular glutamic acid. The knowledge on the membrane permeability of glutamic acid is successfully exploited for increased industrial production of glutamic acid.
Production of Glutamic Acid-Requirements and Influencing Factors:
The industrial production of glutamic acid is influenced by carbon sources, nitrogen sources, growth factors, pH and O2 supply. The relevant aspects are briefly described.
Carbon sources:
Either refined (glucose, sucrose, fructose, maltose) or unrefined (sugar beet molasses, sugar cane molasses) carbon sources are used. In countries like Japan, acetate (inexpensive) is utilized. Other substrates like alkanes, ethanol and methanol are less frequently used.
Nitrogen sources:
The concentration of ammonia is very crucial for converting carbon source to glutamic acid. However, high concentration of ammonia inhibits the growth of the organisms. In the beginning of fermentation, ammonium salts and a low concentration of ammonia are added.
During the course of fermentation, ammonia in aqueous solution is continuously fed. In this way, pH can be controlled, besides continuous supply of nitrogen source. Sometimes, urea is also used as a nitrogen source, since glutamic acid-producing bacteria possess urease that can split urea and release ammonia.
Growth factors:
Biotin is an important growth factor and its concentration in the medium is influenced by the carbon source. For instance, a supply 5 µg of biotin per liter medium is recommended if the carbon source is 10% glucose; while for acetate as the carbon source, the biotin requirement is much lower (0.1-1.0 µg/l). Addition of L-cysteine in the medium is recommended for certain strains.
Supply of O2:
O2 supply should be adequately and continuously maintained. It is observed that a high O2 concentration inhibits growth of the organisms while a low O2 supply leads to the production of lactic acid and succinic acid. In both instances, glutamic acid formation is low.
Process of Production and Recovery:
Some important information on the production of glutamic acid by Brevibacterium divaricatum is given below.
Carbon source – Glucose (12%)
Nitrogen source – Ammonium acetate (0.5%)
pH – 7.8
Temperature – 38°C
Period for fermentation – 30-35 hours
Yield of glutamic acid – 100 g/l medium.
A schematic representation of glutamic acid production plant is shown in Fig. 26.3. As the fermentation is complete, the cells are separated, the culture broth is passed through anion exchanger. The glutamic acid bound to the resins is eluted in NaOH, while the ammonia released can be reused. With NaOH, glutamic acid forms monosodium glutamate (MSG) which can be purified by passing through anion exchanger. MSG can be subjected to evaporation and crystallization.
Type # 3. L-Lysine:
Lysine is present at a low concentration in most of the plant proteins. Being an essential amino acid, supplementation of plant foods with lysine increases their nutritional quality.
L-Lysine is predominantly produced by Corynebacterium glutamicum and to some extent by Brevibacterium flavum or B. lactofermentum.
Biosynthesis of L-lysine:
The pathway for the synthesis of L-lysine is complex, and an outline of it is depicted in Fig. 26.4. This metabolic pathway is also involved in the formation of 3 other amino acids, namely methionine, threonine and isoleucine.
As the glucose gets oxidised by glycolysis, phosphoenol pyruvate and pyruvate are formed. Both these metabolites can be converted to oxaloacetate, a key component of citric acid cycle. On transamination, oxaloacetate forms aspartate. The enzyme aspartate kinase converts aspartate to aspartyl phosphate which later forms aspartate semi-aldehyde.
Aspartate semi-aldehyde has two fates—the biosynthesis of lysine and formation of 3 other amino acids (methionine, threonine and isoleucine). When homoserine dehydrogenase acts on aspartate semi-aldehyde, it is diverted for the synthesis of 3 amino acids. The enzyme dihydrodipicolinate synthase converts aspartate semi-aldehyde (and pyruvate) to piperideine 2, 6-dicarboxylate.
There are two distinct enzymes succinylase variant (catalyses 4-step reaction) and dehydrogenase variant (catalyses a single step reaction) that can convert piperideine 2, 6-dicarboxylate to D, L-diaminopimelate which later forms L-lysine.
Regulation of L-lysine Biosynthesis:
The following are the regulatory processes in the production of lysine (Fig. 26.4).
Aspartate kinase:
This enzyme is controlled by feedback inhibition of the end products. Three isoenzymes of aspartate kinase have been identified-one repressed by L-methionine, the second one repressed by L-threonine and L-isoleucine, and the third one being inhibited and repressed by L-lysine. The amino acid sequence and structure of aspartate kinase have been elucidated. And by genetic manipulations, it has been possible to create mutants (of aspartate kinase) that are insensitive to feedback regulation by L-lysine.
Dihydrodipicolinate synthase:
This enzyme competes with homoserine dehydrogenase to act on aspartate semi-aldehyde. Overexpression of dihydrodipicolinate synthase has been shown to increase the production of L-lysine.
Succinylase and dehydrogenase variants:
The conversion of piperideine 2, 6-dicarboxylate to D, L-diaminopimelate is carried out by these two enzymes. At the start of the fermentation, dehydrogenase variant predominantly acts, and later succinylase variant comes into picture for the biosynthesis of L-lysine.
Role of D, L-diaminopimelate:
This amino acid, an immediate precursor for the synthesis of L-lysine, is also required for the synthesis of a tripeptide (L-Ala-ƴ-D-Glu-D, L-Dap) which is part of the peptidoglycan of cell wall. The activities of both the enzymes (succinylase and dehydrogenase) that form diaminopimelate (Dap) are important for the production of L-lysine and for the proper formation of cell wall structure.
Improved Production Strains:
Based on the biosynthetic pathway and the regulatory steps certain improvements have been made in the strains of C. glutamicum and B. flavum for overproduction of lysine.
i. Mutant organisms resistant to lysine antimetabolites (e.g. b-amino ethyl-L-lysine).
ii. A mutant strain with an altered enzyme aspartokinase, so that it is not regulated by end product inhibition.
iii. A strain with a decreased homoserine dehydrogenase activity (so that diversion for the synthesis of methionine, threonine and isoleucine is minimised).
iv. A strain with reduced citrate synthase activity (to lower the occurrence of citric acid cycle).
Release of L-Lysine:
The export or release of L-lysine from the cells into the surrounding medium occurs through a lysine-export (LysE) carrier protein. It is a trans membrane protein (mol. wt-25,400) with six segments that participate in lysine transport. The exporter system is very efficient active process to export large quantities of intracellular lysine.
Production Process of L-lysine:
The most commonly used carbon sources for lysine manufacture is molasses (cane or sugar beet), starch hydro lysates or sucrose. The other sources like acetate, ethanol or alkanes are used to a lesser extent. The nitrogen sources are ammonium salts, gaseous ammonia. Protein hydro lysates are added to supply certain amino acids (L-methionine, L-homoserine, L-threonine). The protein hydro lysates also supply growth factors such as biotin.
A time-course graphic representation for the formation of lysine is depicted in Fig. 26.5. As is evident, a continuous supply of glucose (or other sugar) is required for sustained production of lysine. Under optimal fermentation conditions, the yield of lysine (in the form of L-lysine HCI) is 40-50 g per 100 g carbon source.
There are different recovery processes for lysine depending on its application.
i. An alkaline solution containing about 50% L-lysine can be obtained after biomass separation, evaporation and filtration.
ii. A crystalline preparation with 98-99% L-lysine (as L-lysine HCI) can be obtained by subjecting the culture broth to ion-exchange chromatography, evaporation and crystallization.
Both the above grades of lysine are suitable for supplementation of feeds.
Type # 4. L-Threonine:
L-Threonine is manufactured industrially by employing either E. coli or C. glutamicum. With the mutant strains of E. coli, the product yield is better.
Biosynthesis of L-threonine:
The metabolic pathway for the synthesis of L-threonine is depicted in Fig. 26.6. Some of the reactions of this pathway are common for the biosynthesis of L-lysine and methionine, besides isoleucine .Starting with aspartic acid, in a sequence of five steps, threonine is produced.
Regulation:
The regulatory reactions in E. coli for L-threonine biosynthesis have been elucidated. Three isoenzymes of aspartate kinase, separately inhibited by the end products have been identified- one by L-threonine, one by L-methionine and one by L-lysine.
Further, two isoenzymes of homoserine dehydrogenase-one inhibited by L-threonine and other by L-methionine are also known. A gene thrABC that encodes three polypeptides (one polypeptide possesses the activity of kinase and homoserine dehydrogenase, the second homoserine kinase and the third threonine synthase) in E. coli has been identified.
Improved production strains:
The efficiency of the producer strains can be increased by creating E. coli mutants with high-level expression of the gene thrABC. Further, mutants with minimal production of L-isoleucine also result is high yield of L-threonine.
Production Process of L-threonine:
The culture medium containing glucose or sucrose, yeast extract and ammonium salts is adequate for L-threonine production. The sugar feeding has to be continued for good yield (about 60% of the carbon source). The downstream processing for the isolation of L-threonine consists of coagulation of the cell mass (by heat), filtration, and concentration by evaporation, and crystallization.
Type # 5. L-Phenylalanine:
Both E. coli and C. glutamicum can be used for the production of L-phenylalanine. The biosynthetic pathway is quite complex and an outline is shown in Fig. 26.7. An interesting feature is that the same pathway is responsible for the synthesis of all the three aromatic amino acids-tyrosine and tryptophan, besides phenylalanine.
The synthetic pathway commences with the condensation of erythrose 4-phosphate with phosphoenol pyruvate to form deoxyarabinoheptulosonate phosphate (DAHP). DAHP in the next series of reactions is converted to chorismate which can form L-tryptophan. Chorismate mutase converts chorismate to prephenate which forms L-phenylalanine through the participation of prephenate dehydrogenase. Prephenate also serves as a precursor for the synthesis of tyrosine.
The genes responsible for the formation of the regulatory enzymes of L-phenylalanine have been identified. By employing genetic manipulations, strains for improved production of L-phenylalanine have been developed.
Type # 6. L-Tryptophan:
There are different ways of synthesizing L-tryptophan-chemical, enzymatic and fermentation methods. At present, large scale manufacture of tryptophan is carried out by using the enzyme tryptophan synthase of E. coli. Tryptophan synthase combines indole with L-serine to form tryptophan.
Indole is available from petrochemical industries while L-serine can be recovered from molasses during sugar refinement. Mutant strains of E. coli with high activity of tryptophan synthase have been developed for large scale manufacture of tryptophan.
Direct fermentation process:
Tryptophan can also be produced by fermentation employing C. glutamicum, or E. coli. For the biosynthetic pathway, refer Fig. 26.7. Mutant strains of both these organisms have been developed for increased yield of tryptophan.
Mutant Strains for Overproduction L-tryptophan:
The production of tryptophan by C. glutamicum was increased by introducing a second gene encoding anthranilate synthase, a key enzyme in its biosynthesis (Fig. 26.8). Further, genes encoding other important enzymes (deoxyarabinoheptulosonate phosphate synthase, anthranilate phosphoribosyltransferase) were also be modified. The result is that the pathway becomes insensitive to feedback inhibition by end products, leading to an overproduction of L-tryptophan.
Type # 7. L-Aspartic Acid:
There is a growing demand for aspartate, as it is a component of aspartame (an artificial sweetener), besides its use as a food additive, and in pharmaceutical preparations. The preferred method for aspartate production is enzymatic in nature. The enzyme aspartase converts fumarate and ammonia to aspartate. Although this reaction is reversible, aspartate formation is favoured.
The aspartase of E. coli is used. It is a tetramer with a molecular weight 196,000. This enzyme is quite unstable. Immobilization of aspartase in polyacrylamide or carrageenan that enhances the stability of the enzyme is commonly used. Immobilized E. coli cells with good activity of aspartase are also used for aspartate production.