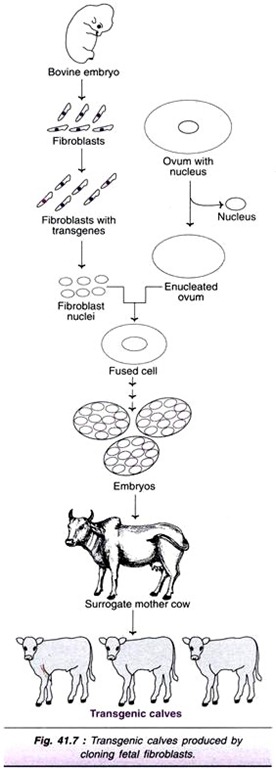
ADVERTISEMENTS:
Read this article to get information about Nucleic Acids, its structure, size, types and significance!
These are important organic substances found in nucleus and cytoplasm. They control the important biosynthetic activities of the cell and carry hereditary information from generation to generation.
Thus, nucleic acids are macromolecules of the utmost biological importance.
ADVERTISEMENTS:
They are associated with the chromosomes and transmit various information to cytoplasm.
All the hereditary (genetic) information of the cell (i.e., all the information necessary to reproduce and maintain a new organism) is stored in coded form in molecules of DNA. DNA is replicated and distributed to daughter cells during cell division, and in this way all the hereditary information accumulated over billions of years of evolution is passed from cell to cell and from one generation of an organism to another.
With the aid of RNA, this information is expressed as specific patterns of protein synthesis. These nucleic acids are of two types: (i) deoxyribonucleic acid (DNA) and (ii) ribonucleic acid (RNA). DNA is the major store of genetic information. This information is transmitted by transcription into RNA molecules, proteins are then synthesized in a process involving translation of the RNA.
ADVERTISEMENTS:
DNA → transcription RNA→ translation Protein
In higher cells DNA is localized mainly in the nucleus as part of the chromosomes. A small amount of DNA is present in the cytoplasm and contained within mitochondria and chloroplasts. RNA is found both in the nucleus, where it is synthesized, and in the cytoplasm, where the synthesis of proteins takes place.
Nucleic acids consist of a sugar (pentose), nitrogenous bases (purines and pyrimidines), and phosphoric acid. A nucleic acid molecule is a linear polymer in which nucleotides are linked together by means of phosphodiester ‘bridges’ or bonds.
These bonds link the 3′ carbon in the pentose of one nucleotide to the 5′ carbon in the pentose of the adjacent nucleotide. Thus the backbone of a nucleic acid consists of alternating phosphates and pentoses. The nitrogenous bases are attached to the sugars of this backbone.
Nucleic acids are basophilic, i.e., stain readily with basic dyes. After a mild hydrolysis the nucleic acids are decomposed into nucleotides.
[I] Deoxyribonucleic acid (DNA):
It forms about 9% part of nucleus as found by spectrophotometric analysis. Chemically it consists of mainly three components: phosphoric acid, sugar, and bases.
1. Phosphoric acid:
It may occur also as phosphate and forms the backbone of DNA molecule along with sugar molecule. It links the nucleotides by joining the deoxyribose (pentoss sugar) of two adjacent nucleotides with an ester-phosphate bond. These bonds connect carbon 3′ in one nucleotide with carbon 5′ in next. This acid is a channel for the chemical energy used by the molecule.
2. Pentoses:
These are of two types: ribose in RNA and deoxyribose in DNA. DNA has one oxygen atom less than that of RNA. The pentose sugar in nucleic acids is always ribose-, in RNA it is D-ribose and in DNA, it is deoxyribose. It is always the OH on C-l carbon which is the point of attachment of the base.
This linked to the 1-nitrogen atom in case of pyrimidines and to 9-nitrogen atom in purines. Both deoxyribose and ribose (pentose sugars of nucleic acids) have a pentagonal ring with five carbons, among which two (i.e., 3′ and 5′) are attached to phosphoric acid and three (Г) to the base.
3. Bases:
ADVERTISEMENTS:
These may be of two types:
(a) Purines and
(b) Pyrimidines.
(a) Purines:
ADVERTISEMENTS:
These are characterised by the presence of two fused benzene rings. They may be adenine (A) and guanine (G). RNA contains uracil (U) instead of thymine. The combination of base plus a pentose, minus the phosphate, forms a nucleoside. For example, adenine is a purine base; adenosine (adenine + ribose) is the corresponding nucleoside, i.e., deoxyadenosine and deoxyguanosine.
(b) Pyrimidines:
These are characterized by the occurrence of single benzene ring. They are thymine (T) and cytosine (C).
Nucleotides:
ADVERTISEMENTS:
Nucleotides are phosphate esters of nucleosides, purine or pyrimidine bases linked to sugars. In the nucleotides, the 3-nitrogen of the pyrimidine bases or the 9-N of the purine bases is attached to the 1-carbon atom of the sugar, and the phosphoric acid residue is attached to the 5′ carbon atom of the sugar.
Thus nucleotides of purines are deoxyriboadenylic acid and deoxyriboguanylic acid, and of pyrimidines are deoxyribothymidylic acid and deoxyribocytidylic acid.
[II] DNA:
Purines and pyrimidines are weak bases. Adjacent nucleotides of the nucleic acids are connected by a linkage between the phosphoric acid residue of one nucleotide and the 3′ carbon atom of the sugar on the next nucleotide. Both the bases and sugars have an approximately planar structure. In polynucleotides the planes are oriented with respect to one another at an angle of 70° to 75°.
ADVERTISEMENTS:
In addition to four common bases, a number of unusual bases are found is .DNA. DNA of animal origin contains trace amounts of 5-methy- lcytosine, while large amounts of this base are found in DNA of plant origin. Similarly, 6-methyl aminopurine is found in DNA from bacteria and viruses.
Cytosine in DNA of T-even bacteriophages of E.-coli is replaced by 5-hydroxymethylcytosine, to which glucose or other sugars may be linked at the hydroxyl group. In some viral DNA’s, the rare base 5-hydroxy— methyluracil substitutes for thymine.
[III] DNA base composition:
DNA in living organisms is found as a linear molecules of extremely high molecular weight. For example, in E-coli it is a single circular DNA molecule weighing about 2.7 × 109 daltons and its length is 1.4 mm. In higher organisms the amount of DNA may be several thousand times larger. For example, in a single human diploid cell its total length when fully extended is 1.7 meters.
All the genetic information of a living organism is stored in its linear sequence of the four bases. Therefore, a four letter alphabet (A, T, G, C) must code for the primary structure (i.e., number and sequence of 20 amino acids) of all proteins. The base composition vary from one species to another, but in all cases amount of adenine is equal to amount of thymine (A=T).
Similarly amount of cytosine is equal to guanine (C=G). Consequently, total quantity of purines equals the total quantity of pyrimidines (i.e., A+G=C+T). On the other hand, AT/GC ratio varies considerably between species.
[IV] DNA is double helix:
On the basis of X-ray diffraction data of Wilkins and Franklin, Watson and Crick (1953) proposed a model for DNA structure. It is composed of two right-handed helical polynucleotide chains that form a double helix around the same central axis. The two strands are antiparallel, meaning that their 3′, 5′ phosphodiester links run in opposite directions. The bases are stacked inside the helix in a plane perpendicular to the helical axis.
ADVERTISEMENTS:
The two strands are held together by hydrogen bonds present between pairs of bases. Since there is a fixed distance between two pentose sugars in the opposite strands, only certain base pairs can fit into the structure.
As shown in figure 5 two hydrogen bonds are formed between A and T, three are formed between С and G, therefore a CG pair is more stable than AT pair. In addition to hydrogen bonds, hydrophobic interactions established between the stacked bases are important in maintaining the double helical structure.
The axial sequence of bases along one polynucleotide chain may vary considerably, but on the other chain the sequence must be complementary, as given below —
Because of this property, order of bases on one chain, the other chain is complimentary. During duplication the two chains dissociate and each one serves as a template for the synthesis of a new complementary chain.
ADVERTISEMENTS:
[V] Separation of DNA strands:
DNA double helix is preserved by weak interactions (i.e., hydrogen bonds and hydrophobic interactions between stacked bases); two strands may be separated by heating or alkaline pH. This separation is called melting or denaturation of DNA. The melting point depends on AT/GC ratio. Breakage of GC pairs needs higher temperature to that of AT pairs.
If DNA is cooled slowly after denaturation, double helical conformation will be restored. This process is called renaturation or annealing and this is the base-pairing properties of nucleotides.
DNA renaturation can be used to estimate the size (number of nucleotides) of the genome of a given organism. A large genome (e.g., calf) take more time to reanneal than a small genome (e.g., E. coli). This is because the individual sequences take longer time to find the correct partners.
Single stranded DNA will also anneal to complimentary RNA, resulting in a hybrid molecule in which one strand is DNA and the other is RNA. Molecular hybridization is a very powerful method for characterizing RNAs since RNA; molecule will hybridize only to DNA from which it was transcribed.
[VI] Ribonucleic acid (RNA):
RNA is present in considerable amounts in the nucleolus and is also found in small amounts on chromosomes. The major part of the cells RNA is in the cytoplasmic ribosomes. A small amount of RNA is also present in mitochondria and chloroplasts.
Transfer RNA and mRNA are present in solution in the cytoplasmic matrix unless affixed to the ribosomes. The RNA content of nucleus and cytoplasm varies with activity cycles of the cell. The cytoplasmic RNA increases in quantity during cell growth preceding mitosis and is partitioned equally between the daughter cells.
RNA accumulates in both nucleus (especially in nucleolus) and cytoplasm during high metabolic activity or growth, as in regenerating nerve cells, active neurons, gland cells, cells infected with virus and tumor cells. Actively metabolizing yeast cells contain a large amount of RNA, but starved yeast cells have little RNA. Infact, starved cells in general show RNA depletion.
RNA also varies with other physiological conditions such as lack of oxygen and presence of metabolic poisons. RNA is labile in dividing cells and also in active cells that are not dividing.
[VII] Structure of RNA:
RNA is a long-chain molecule built up of repeating nucleotide units linked by 3′ to 5′ phosphate diester bonds. Sugar component of RNA is ribose and three out of four bases, adenine, guanine and cytosine are the same as in DNA, and the fourth base is uracil in place of thymine of DNA, Uracil has one methyl group less.
Nucleotides:
RNA nucleotides are formed from pentose sugar ribose, phosphoric acid and either adenine guanine, cytosine or uracil (U). Nucleotides are regarded as phosphorylated derivatives of nucleosides. Nucleosides are combinations of a nitrogenous base and a pentose sugar without an attached phosphate group.
A nucleotide unit consists of a molecule of sugar, a base and a phosphoric acid. A single nucleic acid contains a large number of nucleotide units consisting of high molecular weight (about 8,000,000).
Nucleotides are the monomeric units of the nucleic acid macromolecule. The nucleotides result from the covalent bonding of a phosphate and a heterocyclic base to the pentose. Within the nucleotide, the combination of a base with the pentose forms a nucleoside.
For example, adenine is a purine base; adenosine (adenine+ ribose) is the corresponding nucleoside, and adenosine monophosphate (AMP), adenosine diphosphate (ADP) and adenosine triphosphate (ATP) are nucleotides. Nucleotides, thus constitute the building blocks of nucleic acids and they are also used to store and transfer chemical energy.
Polynucleotide:
Nucleotides are joined together to form a polynucleotide chain by a covalent linkage between the phosphoric acid residue of one nucleotide and 3′ carbon of the sugar on the next nucleotide. This linkage is often called a 3′, 5′ phosphodiester bond, because the phosphate is esterified to two OH groups, one attached to the 3′ carbon and one attached to the 5′ carbon.
The backbone of a polynucleotide chain thus consists of alternating sugar and phosphate units.
The sequence of nucleotides in DNA and RNA is the key to their genetic functions, just as the sequence of amino acids determines the biological activity of a particular protein. Even though both DNA and RNA are usually composed of only four different nucleotides, the number of possible sequences of nucleotides is enormous in a large polymer.
RNA usually exists as a single-stranded polynucleotide chain and have no regular helical configuration. The linear chain is thought to be folded in many ways, with certain nucleotides pairing off and forming short double-stranded regions.
[VIII] Kinds of ribonucleic acid:
The ribonucleic acids are of three types —
1. Messenger RNA:
This ribonucleic acid is of nuclear origin and conveys genetic information from DNA in the nucleus to the ribosomes in the cytoplasm, where amino acids become grouped to form proteins.
2. Transfer or adapter RNA:
It is another important type of ribonucleic acid which is present in the cytoplasm, helping there in protein synthesis. It has been recently found that t-RNA originates from nucleus near the nucleolar region.
3. Ribosomal RNA:
This ribonucleic acid is the major component of cytoplasmic particles called ribosomes. Ribosomal RNA comprises up to 80% of the cellular RNA of Escherichia coli. It is the site of amino acids union.
For detailed structure see chapter-Protein synthesis.
[IX] Significance of nucleic acids:
Deoxyribonucleic acids and ribonucleic acids are the key centres which control all the metabolic activities of cell and in turn the whole organism.
(1) If there occurs any deficiency in the DNA amount, nucleus loses its capacity to support adenosine triphosphate (ATP) synthesis.
(2) Nucleus also becomes inefficient to incorporate amino acids into proteins.
(3) Besides, DNA is the main genetic material constituting genes and chromosomes which carry hereditary information from generation to generation. DNA helps in the RNA synthesis in the cell. If the loops of amphibian oocytic chromosome (lamp brush) are exposed to actinomycin (which has the property to fuse with DNA and thereby causing decrease in DNA amount), RNA synthesis is inhibited.
(4) Recently, McConnell and Cameron (1968) have produced the evidence that RNA amount increases the intelligence and learning capacity of men.
Molecular arrangement of components in nucleic acids:
In deoxyribonucleic acid (DNA), nucleotides are arranged in the form of helixes or chains spirally coiling around each other. According to Watson and Crick (1962), DNA consists of two helixes coiled about each other. The chain of each helix is made of sugar and phosphate group.
These two helixes are interconnected by the bases through hydrogen bonds. Generally one purine becomes attached with one pyrimidine to form base connection between the chain. Thus, adenine along with thymine, and cytosine along with guanine becomes connected with the sugar molecule of chain alternately.
The direction of one helix is opposite to the other. In DNA one helix serves as a template for the formation of complementary helix, i.e., adenine in one helix forms the thymine in new helix and similarly cytosine effects the formation of guanine in new helix.
In ribonucleic acid (RNA), the nucleotides are not arranged in double helical model but for the most part RNA exists as a single strand. Sometimes it may form also smaller helices in some parts due to folding and convolutions. These secondary helical structures in RNA are regular according to recent research. In the formation of helix, bases become hydrogen-bonded like DNA except uracil substitutes thymine.