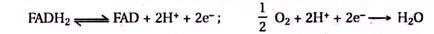ADVERTISEMENTS:
In this article, we will discuss about the nucleoside and nucleotide.
A nucleoside is composed of two components:
(a) Nitrogenous base and
ADVERTISEMENTS:
(b) Five-carbon sugar (pentose).
1. Nitrogenous bases:
The nitrogenous bases are derivatives of two parent heterocyclic compounds, i.e., purine and pyrimidine.
2. Pentose Sugars:
ADVERTISEMENTS:
The pentose sugar present in the nucleic acids is ribose in RNA and deoxyribose in DNA.
A nucleoside is formed by the linkage of the nitrogenous base to the -OH group at the first carbon of the pentose sugar through an N-glycosyl linkage. The binding of the two nitrogenous bases to the -OH group at the first position in ribose sugar differs. The purine bases are linked through the nitrogen present at the ninth (9th) position and the pyrimidine bases are linked by the nitrogen present at the position one (1).
The various nucleosides thus formed are:
Purines:
Adenine + Pentose = Adenosine
Guanine + Pentose = Guanosine
Pyrimidine’s:
ADVERTISEMENTS:
Cytosine + Pentose = Cytidine
Thymine + Pentose = Thymidine
Uracil + Pentose = Uridine
Nucleotide:
ADVERTISEMENTS:
A nucleotide is formed by esterification of phosphoric acid to the —OH group present at the fifth (5th) position of the pentose sugar in a nucleoside.
It can be observed in the nucleotide structures that, there are two cyclic rings—one pentose and the other nitrogenous base. While referring to any member in a particular ring, the number of the member will overlap in both the rings. Hence, in order to avoid confusion, and for convenience the members in the nitrogenous bases and the sugar are numbered differently.
The members of the purine ring are numbered from 1 to 9 and that of pyrimidine ring from 1 to 6. On the other hand, the members of the pentose sugar are numbered as 1′, 2′, 3′, 4′ and 5′ (read as one prime, two prime, three prime, four prime and five prime).
ADVERTISEMENTS:
Purines:
Adenine + Ribose + Phosphoric acid → Adenylate (present in RNA)
Adenine + Deoxyribose + Phosphoric acid → Deoxyadenylate (present in DNA)
Guanine + Ribose + Phosphoric acid → Guanilate (present in RNA)
ADVERTISEMENTS:
Guanine + Deoxyribose + Phosphoric acid → Deoxyguanilate (present in DNA)
Pyrimidine’s:
Cytosine + Ribose + Phosphoric acid → Cytidylate (present in RNA)
Cytosine + Deoxyribose + Phosphoric acid → Deoxycytidylate (in DNA)
Thymidine + Deoxyribose + Phosphoric acid → Deoxythymidylate (in DNA)
Uracil + Ribose + Phosphoric acid → Uridylate (present in RNA)
ADVERTISEMENTS:
Polynucleotide:
A polynucleotide is formed by linkage of nucleotides through phosphodiester bonds formed between the hydroxyl group at 3′-carbon of the sugar of one nucleotide and the phosphate at the 5′-carbon of the other nucleotide. A polyribonucleotide forms an RNA and a polydeoxyribonucleotide forms a DNA.
Thus every polynucleotide will have a free unbounded 3′ carbon (free -OH) at one end called the 3′-end, and at the other end the 5′ carbon containing the phosphoric acid is free and this end is called the 5′-end of that polynucleotide. While representing the nucleotide base sequence of DNA or RNA on paper it is represented in the 5′ → 3′ direction—
5′ -A-A-T-C-T-G-G-C-A-C-T- 3′
Nucleotides are linked together in a specific sequence, specific for a species DNA or RNA. The nucleic acids provide the script for everything that occurs in a cell, i.e., the amino acid sequence of every protein in a cell is specified by the cooperation of different RNA which in turn are specified by that cell’s DNA. Thus the structure of every protein, and ultimately of cell constituents, is a product of information programmed into the nucleotide sequence of a cell’s nucleic acid.
Nucleotides of biological importance:
ADVERTISEMENTS:
Nucleotides in addition to the formation of nucleic acids have many other functions in the cell. They act as energy barriers, they are components of coenzymes, act as chemical messenger, etc.
Structure and function of a few biologically important nucleotides are mentioned below:
1. Adenosine-Triphosphate (ATP):
Adenosine triphosphate or ATP is generally known as energy currency of the cell, as it takes up energy from energy yielding processes and donates it to energy requiring cell processes. It is composed of the purine base adenine, attached to the first carbon of the ribose sugar and the 5th carbon of the sugar contains three phosphate groups.
The first phosphate is bonded through an ester linkage, whereas the other two phosphates are linked through anhydride linkage. Hydrolysis of each of the anhydride linkage yields energy of 7.4 kcal/mol, hence they are known as high energy phosphate bonds. The hydrolysis of the third phosphate bond (ester linked, alpha bond) yields only 4 kcal/mol of energy. Hence it is not a high energy bond.
2. Adenosine-Diphosphate (ADP) and Adenosine-Monophosphate (AMP):
ADP and AMP are the hydrolysis products of ATP. They take part in a variety of cellular reactions. ADP participates in energy yielding reactions, thereby capturing the energy in the form of high energy bond and getting converted to ATP.
ADVERTISEMENTS:
Example:
Phosphoenol pyruvate + ADP — PYRUVATE KINASE → Pyruvate + ATP
ADP is converted to ATP by the energy released during electron transport chain in the mitochondrial respiration. ADP and AMP act as positive allosteric enzyme modulator for many enzymes of energy yielding reactions.
3. Guanidinc-Triphosphate (GTP), Cytidine-Triphosphate (CTP), Uridine-Triphosphate (UTP):
GTP, CTP and UTP act as energy currency of the cells, but in a limited number of reactions.
To quote an example for each:
Succinyl-CoA + GDP — SUCCINATE THIOKINASE → Succinic acid + GTP
Glucose-1-phosphate + UTP — PYROPHOSPHORYLASE → UDP-Glucose + pp
Diacylglycerol + CTP — KINASE → CDP-diacylglycerol + pp
4. Adenosine 3′, 5′-Cyclic Monophosphate (cAMP) and Guanosine 3′, S’-Cyclic Monophosphate (cGMP):
cAMP and cGMP act a second messengers formed from ATP and GTP by the action of the enzymes adenylate cyclase and guanilynate cyclase respectively. They are formed in response to hormones like epinephrine and serves regulatory function within the cell (e.g., Activation of glycogen phosphorylase). Structurally both are ribonucleotides of adenine and guanine respectively, wherein the phosphate present at the 5′ position is bonded to the 3′ — OH, thereby forming an additional cyclic ring.
5. S-Adenosyl-Methionine (adoMET or SAM):
adoMET is synthesized from ATP and methionine by the action of the enzyme methionine adenosyl transferase, wherein the amino acid methionine is attached to the 5′ end of the adenosine nucleoside. adoMET is used for methyl group transfers in the synthesis of amino acids and nucleotide metabolism.
6. Nicotinamide Adenine Dinucleotide (NAD), Nicotinamide Adenine Dinucleotide Phosphate (NADP) and Flavine Adenine Dinucleotide (FAD):
NAD, NADP and FAD act as coenzymes in many oxidation-reduction reactions. They take up electrons and H+, and get converted to NADH + H+, NADPH and FADH2 during oxidative reaction and in turn give up electrons and H+ during reductive reactions.