ADVERTISEMENTS:
Let us make an in-depth study of the nucleic acids. The below given article will help you to learn about the following things:- 1. DNA 2. Structure of DNA 3. Chemical Composition of DNA 4. Nitrogen Bases 5. Nucleosides and Nucleotides 5. Polymerization 6. Genetic Information in DNA 7. Chargaff Equivalence Rule 8. Types of DNA and Others.
In 1868 Meischer isolated a substance from the nucleus of pus cells. By digesting pus cells in HC1, he obtained a pure material and named it nuclein. Nuclein had strong acidic properties and contained considerable amount of phosphorus.
In 1889 Altmann coined the term nucleic acid. Later existence of two types of nucleic acid, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) was established.
ADVERTISEMENTS:
The present knowledge about the structure of DNA is the result of research by great scientists like Chargaff, Stendel, Levene, Todd, Wilkins, Rosalind. Franklin, Astbury, Watson, Crick, Hargobind Khorana and many others.
All living organisms possess, nucleic acids. Nucleic acids are macromolecules of utmost biological importance. Nucleic acids possess all the information needed for an organism’s cell structure, function, development and reproduction.
DNA:
DNA is mainly present in the chromosomes in the nucleus. A small amount of DNA is present in the mitochondria and chloroplasts. Mitochondria and chloroplasts are self- replicating bodies.
DNA molecule occupies central position among biological macromolecules. It is a storehouse of genetic information. Nucleotides sequences of DNA encode proteins and enzymes, which directly or indirectly control the synthesis of all cellular components.
ADVERTISEMENTS:
DNA has an excellent mechanism for stable storage of genetic information. The enzymes that synthesize DNA, faithfully copy DNA molecules containing millions of bases. They perform this function with high degree of accuracy and speed. DNA is the only macromolecule for which repair mechanism exists if any damage is caused to it.
Structure of DNA:
DNA molecules are largest molecules of the cell, much larger than those of proteins. DNA is found in all animal and plant cells, prokaryotes and most of the viruses. In addition, it is also found in mitochondria and chloroplasts.
Chemical Composition of DNA:
DNA is made up of numerous monomer units called deoxyribonucleotides. Each deoxyribonucleotide consists of three components.
1. Pentose sugar – deoxyribose
2. A nitrogenous base – Purine or Pyrimidine
3. Phosphorus molecule.
Pentose sugar:
In DNA, the pentose sugar is deoxyribose. A similar pentose sugar ribose is present in RNA. The only difference between the two is the absence of hydroxyl group at position number two of the sugar ring in DNA. Therefore, it is called 2′-deoxyribose. This makes DNA more stable. Deoxyribose can be stained with Feulgen stain.
Nitrogen Bases:
Nitrogen bases in DNA are of two kinds, Purine and Pyrimidine.
Purine:
Purines are dicyclic and have fused five and six member rings. Purines are of two kinds, Adenine (A) and Guanine (G).
Pyrimidines:
Pyrimidines are monocyclic and have a six member ring. Pyrimidines are of two kinds – Thymine (T) and Cytosine (C).
In RNA, thymine is replaced by uracil (U). The only difference between uracil and thymine is the presence of a methyl subunit at position C-5 in thymine.
In this way DNA consists of four bases A, G, C, T and RNA has A, G, C, U.
ADVERTISEMENTS:
The nitrogen base is linked to the sugar molecule by a glycosidic bond. The bond is formed between the first carbon atom of the sugar and nitrogen at position 1 in the case of pyrimidine and at position 9 in the case of purine.
Phosphate Group:
Phosphate group is attached to the 5′-carbon of deoxyribose sugar of one nucleotide and 3′-carbon of the deoxyribose sugar of next nucleotide. Phosphate group provides strong negative charge to the nucleic acid. The bond between phosphate and deoxyribose sugar is phosphodiester bond.
Nucleosides and Nucleotides:
Within the structure of the nucleic acid, a nitrogen base is linked to sugar to form nucleoside. Nucleosides are called deoxyribouncleosides. Four kinds of nucleosides are formed in DNA.
Deoxyribose + Adenine → Deoxyadenosine
Deoxyribose + Guanine → Deoxyguanosine
Deoxyribose + Cytosine → Deoxycytidine
Deoxyribose + Thymine → Deoxythymidine
Purine nucleosides are suffixed by sine while pyramidine nucleosides are suffixed by dine.
ADVERTISEMENTS:
Nucleotides:
Nucleotides are phosphoric acid esters of nucleosides with phosphorous at position C-5 of the sugar of one nucleoside and at C-3 of the next nucleoside. Nucleoside is joined to phosphorus by phosphodiester bands.
Four nucleotides are:
Deoxyribonucleotide triphosphates dATP, dGTP, dCTP and dTTP are present in the cytoplasmic pool. The DNA polymerase enzyme acts only on triphosphates.
Polymerization:
ADVERTISEMENTS:
Both strands of DNA are polynucleotide strands. Nucleotides undergo polymerization to form long chain of nucleotides. Nucleotides are repeating units. They are joined end to end to form chain or strands. Adjacent nucleotides are linked to each other – phosphodiester bonds between 5′-C of one nucleotide and 3′-C of the next nucleotide.
Each strand may possess thousands to millions of nucleotides within a single macromolecule. Each – DNA strand has free 5′-phosphate at one end and free 3′-hydroxyl at the other end.
Phosphodiester linkages provide polarity to DNA. Each strand has a polarity. One polynucleotide chain is written 3′ → 5′ direction upward or 5′ → 3′ direction downward. The other strand is antiparallel By convention the sequence of nucleotides is written in 5′ → 3′ direction from the left.
Nucleotides are building blocks of nucleic acids. Alternating series of sugar-phosphate- sugar molecules form the backbone of the each strand linked by phosphodiester bonds. During nucleic acid synthesis, the chain elongation takes place by addition of nucleotides one by one.
The 5′-end of the triphosphate of one nucleotide reacts with 3′-OH group of sugar of the next nucleotide. A phosphodiester bond is formed between first phosphate of incoming nucleotide with 3′-OH of the sugar of next nucleotide. The other two phosphate groups are released as the single pyrophosphate molecule.
Arthur Korenberg demonstrated that nucleotide building blocks for DNA are energy rich d ATP, dGTP, dCTP and dTTP.
Order of bases along polynucleotide chain is irregular. This unique irregularity along with long length is the basis for the enormous information stored in DNA. Thus arrangement of bases is specific for every gene.
Genetic Information in DNA:
The genetic information in DNA is conveyed by the sequence of four nucleotides. The genetic specificity exists in the linear sequence of four nucleotide building blocks. The four bases A, G, T and C can carry infinite number of genetic messages (4N).
Chargaff Equivalence Rule:
In 1950 Erwin Chargaff analysed and measured the base composition of DNA from different organisms by using paper chromatography. Exact ratios of of the four nucleotides vary from species to species. He discovered that in all DNAs the amount of purine was equal to the amount of pyrimidine. Thus A + G = C + T. Further, the amount of Adenine (A) was equal to the amount of thymine (T) and amount of cytosine (C) was equal to the amount of guanine (G).
However, A + T/G + C ratio varies from species to species. A + T/G + C ratio in human sperm is 1.62, in yeast it is 1.79 and in bacteriophages T2 it is 1.86. In higher plants and animals generally A – T composition is high whereas in lower plants and animals G – C composition is high.
X-Ray crystallographic studies of DNA:
By X-ray crystallographic studies of DNA, Astbury gave three dimensional structure of DNA. Based on X-ray diffraction data provided by Wilkins, Rosalind Franklin and others, now famous pair of American scientist James Watson and English scientist Francis Crick proposed a model for DNA structure in 1953 in the journal Nature.
Watson and Crick were awarded Nobel Prize for this in 1962. They shared the prize with Wilkins who investigated X-ray diffraction photographs of DNA. Rosalind Franklin missed the Nobel prize because of her death in 1958.
This DNA model called double helix DNA model was a landmark research in the field of biology. It provided explanation for base composition, DNA replication and its biological properties.
Double helix structure of DNA:
Watson and Crick model of DNA has the following characteristic features:
DNA molecule consists of two polynucleotide strands twisted around each other to form a double helix. The two polynucleotide chains are held together by weak, non-covalent bonds between pairs of bases.
Each nucleotide of a polynucleotide chain consists of phosphate-sugar-base components. Each strand is made up of alternating sugar and phosphate molecules. Bases lie in pairs perpendicular to the axis of the helix. Base pairs are stacked above each other like steps of the staircase.
In double helix molecule of DNA, adenine always pairs with thymine and cytosine with guanine. According to Watson and Crick model hydrogen bonds between nitrogen bases bind the two polynucleotide strands. Adenine is bonded to thymine by two hydrogen bonds A = T and guanine is bonded to cytosine by triple hydrogen bonds C = G.
These hydrogen bonds are weak and can easily break. This enables the two strands to separate easily. Hydrogen bonds between complementary bases contribute to thermodynamic stability of the helix and specificity of base pairing.
Two strands of double helix have complementary sequences:
As the adenine of one strand always pairs with thymine of the other strand and cytosine of one strand always pairs with guanine of the other strand, bases of one strand are complementary to the bases of the other strand. The sequence of bases of one strand dictates the base sequence of the other strand. This is known as Watson-Crick base pairing rule.
To have a constant diameter of DNA double helix, the adenine-thymine and cytosine- guanine pairs are the only ones that can fit the physical dimensions of the helical model. Because purine-purine pair (dicyclic) will be too thick and pyrimidine-pyrimidine pair (monocyclic) too thin.
Following are the other important properties revealed by X-ray diffraction data:
1. The diameter of the helix is 20A or 2 nm. It means the distance between two polynucleotide strands is 20A (1 nm = 10 Angstrom units).
2. The distance between two adjacent base pairs is 3.4 A or 0.34 nm.
3. One complete turn of the helix i.e., 360° takes 34 A or 3.4 nm length of DNA. In this way there are 10 base pairs in one complete turn.
4. Two strands or polynucleotide chains of a helix run in opposite directions, therefore they are antiparallel. One strand has 5′ → 3′ polarity while the other has 3′ → 5′ polarity.
In this way 10 base pairs make one complete turn of 360°. Helical coiling of two strands around the common axis is right handed. Such a DNA is called B-DNA.
The twisting or coiling makes alternating minor and major grooves. The major groove is rich in chemical information and allows regulatory proteins to bind to specific sequences on DNA.
Types of DNA:
DNA is not a rigid molecule. It can exist in different forms depending upon different conditions.
B-Form DNA (B-DNA):
Most of the double helix DNA in the cell has right handed coiling (clockwise) and is called B-form DNA. It has 10 base pairs per turn. Base pairs lie perpendicular to the axis of the helix. It is most stable configuration. When humidity is high (92%) and concentration of ions is low, DNA exists in B-Form. The B-Form of DNA is stable but can change to A, C and D form depending upon the concentration of excess salts and sequence of nucleotides.
A-Form DNA (A-DNA):
A form DNA is also right handed helix. It exists at 75% humidity in the presence of Na+, K+ ions. There are eleven base pairs per turn, which tilt from the axis of helix by 20.2°. It can quickly change to D-form.
C-Form DNA (C-DNA):
C-form of DNA is found at 66% relative humidity in the presence of lithium (Li+) ions. Number of base pairs is 9.33 per turn. The base pairs show a negative tilt of 7.8°.
D-Form DNA (D-DNA):
It is rarely found. There are eight bases per turn. Base pairs show a negative tilt of 16.7°.
Z-Form DNA (Z-DNA):
It is a left handed (sinistral) coiling double helix. The sugar- phosphate backbones are zigzag instead of regular helix. That is why it is called Z-Form. It has 12 base pairs per turn. The Z-form DNA makes one complete turn at 45A as compared to 34 A in B-DNA.
It has 18 A diameter therefore it is thin. Z-form DNA is assumed to play a role in gene regulation. Z-form is found in large number of living organisms including mammals, protozoans and plants.
Single Stranded DNA (ssDNA):
All organisms contain double stranded DNA (ds DNA) except a few viruses such as bacteriophage φ x 174, Provirus phage fd which contains single strand DNA (ds DNA). This single strand DNA is a circular molecule. It becomes double stranded only at the time of replication.
Circular DNA:
Most bacterial chromosomes and many viruses have DNA which is in the form of a closed circle. The two ends of the double helix get covalently sealed to form a closed circle. E. coli has a circular chromosome of about 5 million base pairs.
Plasmids of bacteria are also generally circular. By breaking covalent bonds, the two twisted strands of closed circular DNA, rapidly unwind and separate at the time of replication and transcription. Covalently closed, circular DNA is called ccc DNA. The circular DNA is usually twisted to form super coils. Generally circular DNA is negatively supercoiled.
Enzymes topoisomerases are able to break open the supercoils and open up the circular DNA by making single stranded or double stranded breaks in DNA. Type I topoisomerase makes single stranded breaks and Type II topoisomerase makes double stranded breaks in DNA.
Denaturation and Renaturation:
Because the structure of DNA double helix is kept intact by weak hydrogen bonds, it is possible to separate the two strands. The separation of two strands is called melting or denaturation of DNA. The two hydrogen bonds of A = T can be broken more easily than the three bonds of C = G which require higher temperature. Therefore the temperature at which the two DNA strands become separate depends upon AT/CG ratio. Therefore G : C base pairs contribute more to the stability of DNA than A: T pair.
Similarly, at higher salt concentration of the solution, DNA denatures at higher temperature. This is because two DNA strands contain phosphate groups, which carry a negative charge. These negative charges of two strands repel each other causing their separation. At high salt concentration, the negative charges are shielded by cations, thus providing stability to the helix. Therefore, at low ionic strength, the helix is less stable.
When the temperature is brought down, the complementary strands again form hydrogen bonds to restore original double helix. This process is called renaturation or annealing. It helps to estimate the size of DNA molecule. Time taken to anneal depends upon the length of DNA molecule.
The size of a genome can be estimated by denaturation of DNA and allowing it to re-aneal. The speed of reassociation can be measured in cot units which is concentration x time.
Functions of DNA:
DNA is the genetic material. DNA molecules possess all the information needed for an organism’s cell structure, function, development and reproduction.
It performs the following very important functions:
1. Inheritance:
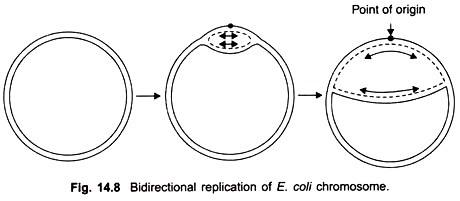
DNA which is the genetic material carries genetic information from cell to cell and to the next generation. This function is performed by DNA replication. It forms exact copies of itself. During DNA replication the two strands separate and each strand functions as a template for the new strand.
In this way one molecule of DNA gives rise to two daughter molecules of DNA both identical to the parent molecule. Each daughter molecule has two strands, one parental (template) and the other new complementary strand.
2. Synthesis of proteins and enzymes:
DNA is transcribed into mRNA and other RNAs. The information contained in the sequence of bases (triplet codons) is translated into proteins and enzymes.
3. DNA molecule is capable of variation:
Variations are caused by mutation and recombination. These variations lead to change in structure and functions of the organisms and lead to evolution of new species.