ADVERTISEMENTS:
Read this essay to learn about the life cycle of ginkgo biloba, explained with the help of suitable diagrams.
Sporophyte of Ginkgo Biloba:
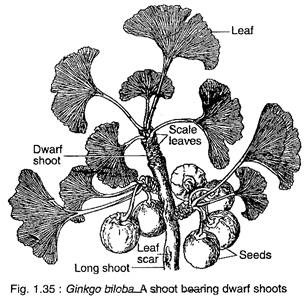
Ginkgo biloba is a tall deciduous tree (up to 30 m height) giving rise to a very irregular pattern of branching. The branching is restricted to the upper part of the stem, thus giving the tree an ex-current pattern of growth. The branches are dimorphic, bearing two types of shoot long shoots and dwarf shoots (Fig. 1.35).
The leaves also show dimorphism, those present on the long shoot are deeply lobed, while those on the dwarf shoots are either not so deeply lobed or may be entire. G. biloba has a tap root system which possesses strong lateral roots that penetrate deep into the soil.
The plants are dioecious. The male and female trees are only distinguishable after the production of reproductive structures on them.
Reproduction:
Ginkgo biloba reproduces sexually. Ginkgo is dioecious. Morphologically, the male and the female plants are indistinguishable before the formation of reproductive organs. However, the male and the female trees can be distinguished cytologically at an early stage.
In the female plant, out of the 24 chromosomes, four are satellite-bearing chromosomes, while in male plant three chromosomes are with satellite. Like cycads, the reproductive structures of Ginkgo are most primitive among living seed plants.
Male Strobilus:
ADVERTISEMENTS:
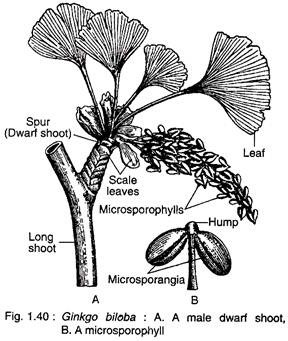
Male strobili develop in the axil of leaves or on the sides of petiole bases present on dwarf shoots (Fig. 1.40A). The male strobilus is a loose structure and consists of a stalked central axis (2-3 cm in length) on which many microsporophylls are arranged spirally. A male strobilus looks like a catkin inflorescence of angiosperms (Fig. 1.40 A).
Each microsporophyll has a long slender stalk terminating into a knob-like portion or hump. It bears two pendent microsporangia (Fig. 1.40B). The hump is always provided with a mucilage duct.
The morphological nature of the hump is still an open question. According to some botanists, the hump represents an abortive sporangium and the mucilage cavity represents abortive sporogenous tissue. In rare instances, a microsporophyll bears three sporangia.
The microsporangia develops from a hypo- dermal archesporial cell of the microsporophyll. A mature microsporangium consists of a multi- layered wall, tapetum and microspore mother cells.
It is important to note that 1-3 hypodermal layers develop fibrous thickening representing an endothecium as in angiosperm. In this point, Ginkgo differs from other gymnosperms where the outermost layer of the microsporangium develops fibrous thickening representing an exothecium.
Inside the sporangium, each microspore mother cell produces four microspores or pollen grains by meiotic division. The pollen grain is oval-shaped with monosulcate aperture. Each grain is bounded by two concentric wall layers: the outer thick exine and the inner thin intine.
The exine is not continuous, being absent at the top apertural region. The dehiscence of sporangia takes place by longitudinal slits. At the time of dehiscence the sporangia are pulled apart due to the shrinkage of mucilage cavity of the hump.
Female Strobilus:
ADVERTISEMENTS:
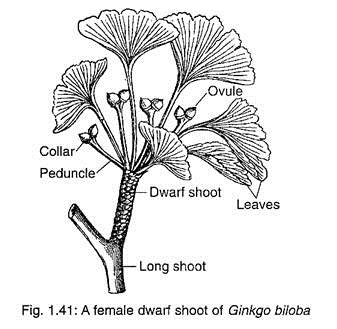
The female strobili develop in the axil of a leaf or a scale leaf present on dwarf shoots (Fig. 1.41). The female strobili are very much reduced structures. Each strobilus consists of a long stalk or peduncle that bifurcates at its apex and each branch usually bears a single sessile ovule (Fig. 1.42A).
Out of the two ovules, one remains viable while the other aborts at an early stage. Sometimes, both the ovules become viable and develop into seeds. The base of each ovule is surrounded by a fleshy cup called collar.
Most of the scientists believed that the female strobilus of G. biloba is a very reduced structure and the collar represents a reduced megasporophyll. Sometimes, the collar may grow into a leafy shoot — it supports this hypothesis.
ADVERTISEMENTS:
Moreover, the peduncle bearing two ovules has four vascular traces, two in each ovule (Fig. 1.42B), thus representing the axis of a female strobilus bearing two megasporophylls. Some abnormalities have also been observed where a single peduncle bears many ovules. In such conditions, the number of vascular traces in the peduncle would be twice the number of ovules.
Pankow and Sothman (1967) disagreed over the sporophyll nature of the collar. They considered the ovules of Ginkgo biloba as cauline and terminal on lateral axis. They argued that the collar is devoid of any vascular supply and develops after the formation of integument.
Ovule:
ADVERTISEMENTS:
The ovule of Ginkgo is similar to that of Cycas. The ovules are orthotropous, unitegmic and crassinucellate (with massive nuceller tissue). In the young ovule, the integument is free from the nucellus, except at the chalazal end, while in a matured ovule the integument is fused with the nucellus except at the apex due to the enlargement of the chalazal end (Fig. 1.42B).
The ovule has a massive beak-shaped nucellus (Fig. 1.42A). In a pre-pollinated ovule, the apical nucellar cells degenerates to form a deep pollen chamber and the pollination drop.
The single integument is differentiated into three layers:
The outer fleshy with numerous mucilage cavities, the middle stony and the inner fleshy, eventually becomes papery. The ovule is supplied with two vascular traces which enter into the inner fleshy layer reaching up to the free part of the nucellus.
ADVERTISEMENTS:
Megasporogenesis:
A deeply situated cell of the nucellus is differentiated into a large megaspore mother cell.
The megaspore mother cell is surrounded by a well-developed spongy tissue. At a later stage, the spongy tissue cells adjacent to the enlarging gametophyte lose their cell walls. The megaspore mother cell wall becomes thick due to the development of a double layered wall. Now, the megaspore mother cell undergoes meiotic division to form a liner tetrad of four megaspores.
The upper three megaspores degenerate, while the lowermost one becomes functional.
Gametophyte of Ginkgo Biloba:
The spores (either microspores or megaspores) represent the first phase of gametophyte generation. The microspore or pollen grain is the male gametophyte, while the megaspore is the first stage of female gametophyte which develops into a female gametophyte.
Development of Male Gametophyte before Pollination:
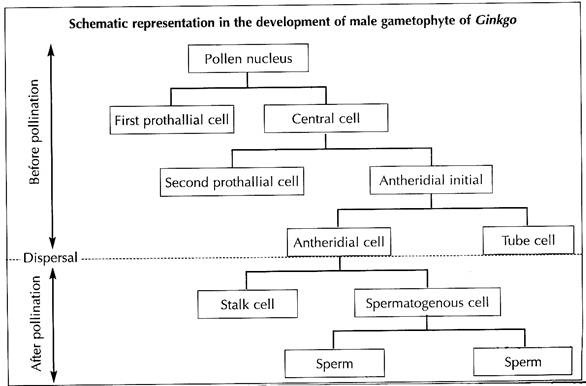
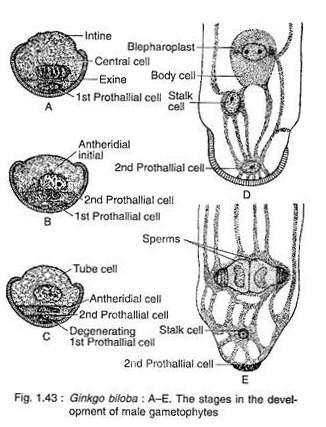
The pollen nucleus undergoes mitotic division to produce a small lens-shaped first prothalial cell towards the proximal end (opposite to the apertural side) and a large central cell on the distal end (Fig. 1.43A). The prothallial cell does not divide, whereas the central cell divides to form a second prothallial cell and an antheridial initial (Fig. 1.43B).
The first prothallial cell is ephemeral, while the second prothallial cell is persistent. The antheridial initial cuts off a small antheridial cell and a large tube cell (Fig. 1.43C). The pollen grains are released from the microsporangium at this 4-celled stage (2 prothallial cells, an antheridial cell and a tube cell).
Development of Male Gametophyte after Pollination:
The further development of male gametophyte starts within a month after pollination. The tube cell of the pollen comes out through the aperture in the form of a pollen tube. The tube proceeds towards the archegonium, penetrating the nucellar tissue of the ovule. The antheridial cell within the pollen tube divides to produce a stalk cell and a spermatogenous (body) cell (Fig. 1.43D).
ADVERTISEMENTS:
The spermatogenous cell enlarges considerably and two blepharoplasts develop at its two opposite end just before fertilisation. The spermatogenous cell divides vertically to form two sperm cells (male gametes) (Fig. 1.43E). The sperms of Ginkgo are very much similar to that of Cycas, except they are smaller in size, slightly elongated and spirally arranged cilia are mostly confined to the apical region.
Development of Female Gamelophyte:
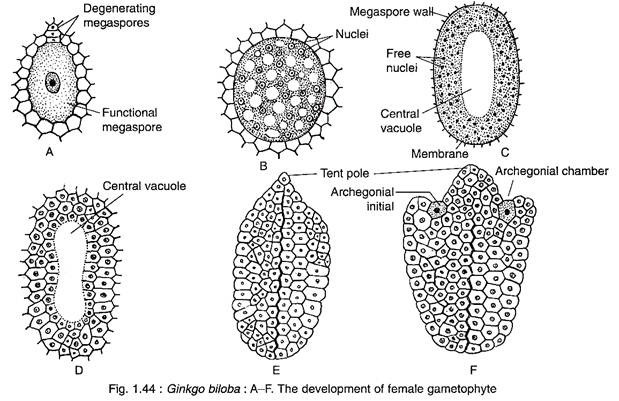
The female gametophyte of Ginkgo develops from the functional megaspore that enlarges considerbly and is surrounded by a thick membrane (Fig. 1.44A).
The nucleus of the megaspore divides mitotically, unaccompanied by wall formation, forming a large number of free nuclei (as much as 8,000 free nuclei) (Fig. 1.44B). The nuclei are restricted to a thin film of cytoplasm at the periphery resulting into the formation of a large central vacuole (Fig. 1.44C).
Thereafter, the cell wall formation begins in a centripetal fashion from periphery inwards (Fig. 1.44D), as a result the vacuole is obliterated. The entire gametophyte becomes cellular and the tissue thus formed is called endosperm (Fig. 1.44E). The endosperm cells are haploid in nature, but some polyploid cells are also formed. The cells that contain 2-3 nuclei during wall-formation are transformed to polyploid cells.
The ultrastructural study of the female gametophyte of Ginkgo shows four different donations according to their food reserves.
They are:
(i) Lipid zone (outer 3—4 layers),
(ii) Starch proteiolipid zone with large vacuolated cells,
(iii) Starch zone consists of large vacuolate cells with peripheral cytoplasm,
(iv) Central zone comprising of less differentiated cells with very little reserve content.
Development of Archegonia:
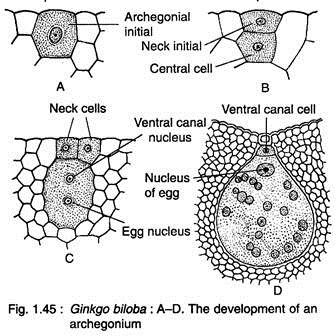
Two to four cells at the micropylar end of the female gemetophyte function as archegonial initials (Fig. 1.44F, 1.45A). Each of these cells divides periclinally to form on outer small primary neck initial and a large central cell (Fig. 1.45B). The primary neck initial divides by two vertical walls at right angles to each other resulting in four neck cells arranged in one tier.
The nucleus of central cell cuts off an upper ephemeral ventral canal cell and a large egg cells (Fig. 1.45C). With regard to ventral canal cell, Ginkgo seems to be more primitive than Cycas where a ventral canal nucleus is present (Fig. 1.45D).
At this stage, the female gametophyte grows upward forming a beak between the two archegonia (Fig. 1.44F, 1.46). The beak extends upward touching the inner surface of the nucellar beak like a pole in a tent and is known as ‘tent pole’.
Pollination:
Like Cycas, Ginkgo is anemophilous i.e., wind-pollinated. At the time of dispersal of pollen grains, the central strand of elongated nucellar cells in the pre-pollinated ovule disorganises followed by the collapse of epidermis resulting into a narrow and deep pollen chamber and pollination drop. The viscous fluid oozes out through the microphyle in the form of pollination drop.
Some of the anemophilous pollen grains are caught in the pollination drop and are brought to the pollen chamber due to the drying-off the fluid. Pollination generally takes place in May. Pollen grains germinate in the pollen chamber, unlike Cycas where they germinate in the intermediary chamber.
The pollen tube comes out through the aperture. The pollen tube branches freely showing a proximal unbranched tube and a much branched haustorial ramification in between the intercellular cells.
Fertilisation:
The sperms along with the pollen tube cytoplasm are released in the archegonial chamber by the rupture of the terminal part of pollen tube. The osmotically rich cytoplasm of pollen tube causes the rupture of neck cells, as a result motile sperms enter into the archegonia. The fertilisation takes place in September i.e., four months after pollination.
The two sperms proceed towards the archegonium with a forward and circular motion, ciliary band forming the posterior end. However, in Cycas, the ciliary band forms the anterior end. The archegonial chamber is filled up with the fertilisation fluid produced by nucellar cells. The ciliary band of the sperm is left behind on the top of the egg cell. The sperm nucleus fuses with the egg nucleus resulting in a zygote.
Embryogeny:
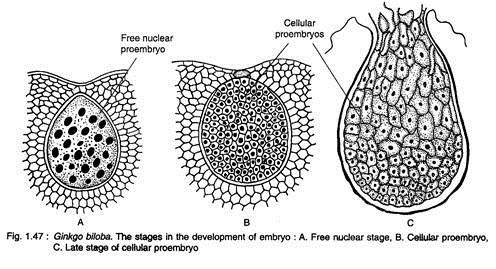
The zygote enlarges considerably and undergoes free nuclear divisions giving rise to 256 nuclei, evenly placed in the cytoplasm of the developing proembryo (Fig. 1.47A). The cell wall formations in the proembryo begin in all the cells simultaneously resulting in a cellular proembryo (Fig. 1.47B). The differentiation of proembryonal cells begins at a later stage.
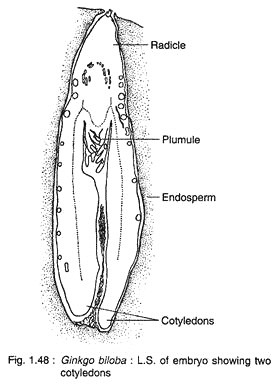
The cells at the micropylar end elongate greatly forming a massive suspensor, while the basal cells (chalazal end) develop into embryonal cells with distinct meristem (Fig. 1.47C). A shoot apex is differentiate at the basal region of the embryo, while a root apex develops at the opposite end. A dicotyledonous embryo (rarely with three cotyledons) with two mesarch bundles is seen in a mature embryo (Fig. 1.48).
Seed:
The most striking feature in Ginkgo is the development of embryo which continues even after the dispersal of seeds. Actually, the major part of the growth of embryo takes place in the detached seeds that are shed before or just after fertilisation. About seven months are required for attaining the maturity of an embryo.
Thus, Ginkgo shows the two-years reproductive cycle where the pollination takes place in spring, fertilisation in early autumn of the same year and the development of embryo continues even after shedding of seeds. The germination of the seed is hypogeal.
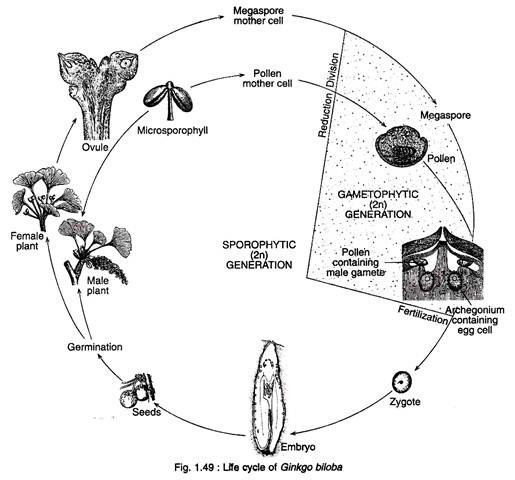
Figure 1.49 shows the life cycle of Ginkgo: