ADVERTISEMENTS:
This article throws light upon the three types of technique used for primary cell culture.
The three types of technique are: (1) Mechanical Disaggregation (2) Enzymatic Disaggregation and (3) Primary Explant Technique.
Primary culture broadly involves the culturing techniques carried following the isolation of the cells, but before the first subculture. Primary cultures are usually prepared from large tissue masses. Thus, these cultures may contain a variety of differentiated cells e.g. fibroblasts, lymphocytes, macrophages, epithelial cells.
ADVERTISEMENTS:
With the experiences of the personnel working in tissue culture laboratories, the following criteria/ characteristics are considered for efficient development of primary cultures:
a. Embryonic tissues rather than adult tissues are preferred for primary cultures. This is due to the fact that the embryonic cells can be disaggregated easily and yield more viable cells, besides rapidly proliferating in vitro.
b. The quantity of cells used in the primary culture should be higher since their survival rate is substantially lower (when compared to subcultures).
c. The tissues should be processed with minimum damage to cells for use in primary culture. Further, the dead cells should be removed.
ADVERTISEMENTS:
d. Selection of an appropriate medium (preferably a nutrient rich one) is advisable. For the addition of serum, fetal bovine source is preferred rather than calf or horse serum.
e. It is necessary to remove the enzymes used for disaggregation of cells by centrifugation.
Techniques for Primary Culture:
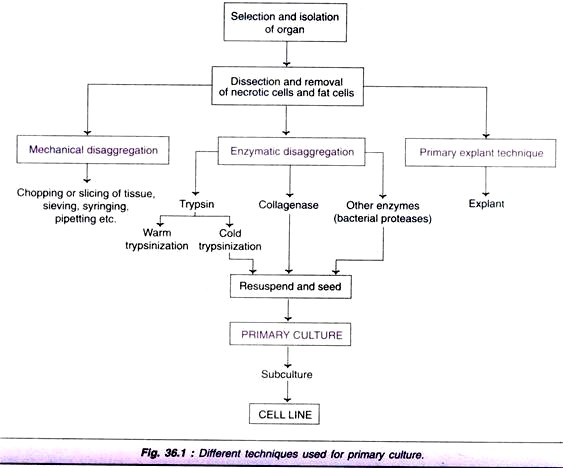
Among the various techniques devised for the primary culture of isolated tissues, three techniques are most commonly used:
1. Mechanical disaggregation.
2. Enzymatic disaggregation.
3. Primary explant technique.
An outline of these techniques is depicted in Fig. 36.1, and the procedures are briefly described:
Technique # 1. Mechanical Disaggregation:
For the disaggregation of soft tissues (e.g. spleen, brain, embryonic liver, soft tumors), mechanical technique is usually employed. This technique basically involves careful chopping or slicing of tissue into pieces and collection of spill out cells.
ADVERTISEMENTS:
The cells can be collected by two ways:
i. Pressing the tissue pieces through a series of sieves with a gradual reduction in the mesh size.
ii. Forcing the tissue fragments through a syringe and needle.
Although mechanical disaggregation involves the risk of cell damage, the procedure is less expensive, quick and simple. This technique is particularly useful when the availability of the tissue is in plenty, and the efficiency of the yield is not very crucial. It must however, be noted that the viability of cells obtained from mechanical techniques is much lower than the enzymatic technique.
Technique # 2. Enzymatic Disaggregation:
ADVERTISEMENTS:
Enzymatic disaggregation is mostly used when high recovery of cells is required from a tissue. Disaggregation of embryonic tissues is more efficient with higher yield of cells by use of enzymes. This is due to the presence of less fibrous connective tissue and extracellular matrix. Enzymatic disaggregation can be carried out by using trypsin, collagenase or some other enzymes.
Disaggregation by trypsin:
The term trypsinization is commonly used for disaggregation of tissues by the enzyme, trypsin.
Many workers prefer to use crude trypsin rather than pure trypsin for the following reasons:
ADVERTISEMENTS:
i. The crude trypsin is more effective due to the presence of other proteases
ii. Cells can tolerate crude trypsin better.
iii. The residual activity of crude trypsin can be easily neutralized by the serum of the culture media (when serum-free media are used, a trypsin inhibitor can be used for neutralization).
Disaggregation of cells can also be carried out by using pure trypsin which is less toxic and more specific in its action. The desired tissue is chopped to 2-3 mm pieces and then subjected to disaggregation by trypsin. There are two techniques of trypsinization-warm trypsinization and cold trypsinization (Fig. 36.2).
Warm trypsinization (Fig. 36.2A):
This method is widely used for disaggregation of cells. The chopped tissue is washed with dissection basal salt solution (DBSS), and then transferred to a flask containing warm trypsin (37° C). The contents are stirred, and at an interval of every thirty minutes, the supernatant containing the dissociated cells can be collected. After removal of trypsin, the cells are dispersed in a suitable medium and preserved (by keeping the vial on ice).
The process of addition of fresh trypsin (to the tissue pieces), incubation and collection of dissociated cells (at 30 minutes intervals) is carried out for about 4 hours. The disaggregated cells are pooled, counted, appropriately diluted and then incubated.
Cold trypsinization (Fig. 36.2B):
This technique is more appropriately referred to as trypsinization with cold pre-exposure. The risk of damage to the cells by prolonged exposure to trypsin at 37°C (in warm trypsinization) can be minimized in this technique.
After chopping and washing, the tissue pieces are kept in a vial (on ice) and soaked with cold trypsin for about 6-24 hours. The trypsin is removed and discarded. However, the tissue pieces contain residual trypsin. These tissue pieces in a medium are incubated at 37°C for 20-30 minutes. The cells get dispersed by repeated pi-pettings. The dissociated cells can be counted, appropriately diluted and then used.
ADVERTISEMENTS:
The cold trypsinization method usually results in a higher yield of viable cells with an improved survival of cells after 24 hours of incubation. This method does not involve stirring or centrifugation, and can be conveniently adopted in a laboratory. The major limitation of cold trypsinization is that it is not suitable for disaggregation of cells from large quantities of tissues.
Limitations of trypsin disaggregation:
Disaggregation by trypsin may damage some cells (e.g. epithelial cells) or it may be almost ineffective for certain tissues (e.g. fibrous connective tissue). Hence other enzymes are also in use for dissociation of cells.
Disaggregation by collagenase:
Collagen is the most abundant structural protein in higher animals. It is mainly present in the extracellular matrix of connective tissue and muscle. The enzyme collagenase (usually a crude one contaminated with non-specific proteases) can be effectively used for the disaggregation of several tissues (normal or malignant) that may be sensitive to trypsin.
Highly purified grades of collagenase have been tried, but they are less effective when compared to crude collagenase. The important stages in collagenase disaggregation, depicted in Fig. 36.3, are briefly described hereunder.
The desired tissue suspended in basal salt solution, containing antibiotics is chopped into pieces. These pieces are washed by settling, and then suspended in a complete medium containing collagenase. After incubating for 1-5 days, the tissue pieces are dispersed by pipetting. The clusters of cells are separated by settling. The epithelial cells and fibroblastic cells can be separated.
Collagenase disaggregation has been successfully used for human brain, lung and several other epithelial tissues, besides various human tumors, and other animal tissues. Addition of another enzyme hyaluronidase (acts on carbohydrate residues on cell surfaces) promotes disaggregation.
Collagenase in combination with hyaluronidase is found to be very effective for dissociating rat or rabbit liver. This can be done by per-fusing the whole organ in situ. Some workers use collagenase in conjunction with trypsin, a formulation developed in chick serum, for disaggregation of certain tissues.
Use of other enzymes in disaggregation:
Trypsin and collagenase are the most widely used enzymes for disaggregation. Certain bacterial proteases (e.g. pronase, dispase) have been used with limited success. Besides hyaluronidase, neuraminidase is also used in conjunction with collagenase for effective degradation of cell surface carbohydrates.
Technique # 3. Primary Explant Technique:
The primary explant technique was, in fact the original method, developed by Harrison in 1907. This technique has undergone several modifications, and is still in use. The simplified procedure adopted for primary explant culture is depicted in Fig. 36.4, and briefly described below.
The tissue in basal salt solution is finely chopped, and washed by settlings. The basal salt solution is then removed. The tissue pieces are spread evenly over the growth surface. After addition of appropriate medium, incubation is carried out for 3-5 days. Then the medium is changed at weekly intervals until a substantial outgrowth of cells is observed. Now, the explants are removed and transferred to a fresh culture vessel.
The primary explant technique is particularly useful for disaggregation of small quantities of tissues (e.g. skin biopsies). The other two techniques mechanical or enzymatic disaggregation however, are not suitable for small amounts of tissues, as there is a risk of losing the cells.
ADVERTISEMENTS:
The limitation of explant technique is the poor adhesiveness of certain tissues to the growth surface, and the selection of cells in the outgrowth. It is however, observed that the primary explant technique can be used for a majority of embryonic cells e.g. fibroblasts, myoblasts, epithelial cells, glial cells.
Separation of Viable and Non-Viable Cells:
It is a common practice to remove the nonviable cells while the primary culture is prepared from the disaggregated cells. This is usually done when the first change of the medium is carried out. The very few left over non-viable cells get diluted and gradually disappear as the proliferation of viable cells commences.
Sometimes, the non-viable cells from the primary cultures may be removed by centrifugation. The cells are mixed with ficoll and sodium metrizoate, and centrifuged. The dead cells form a pellet at the bottom of the tube.
Medical Ethics and Safety Measures in Culture Techniques:
Since the culture techniques involve the use of animal or human tissues, it is absolutely necessary to follow several safety measures and medical ethics. In fact, in some countries there are established legislation/norms for selection and use of tissues in cultures. For example, in United Kingdom, Animal Experiments (Scientific Procedures) Act of 1986 is followed.
The handling of human tissues poses several problems that are not usually encountered with animal tissues. While dealing with fetal materials and human biopsies, the consent of the patient and/his or her relatives, besides the consent of local ethical committee is required. Further, taking any tissue (even in minute quantities) from human donors requires the full consent of the donor in a prescribed format.
The following issues need to be fully considered while dealing with human tissues:
1. The consent of the patient and/or relatives for using tissues for research purposes.
2. Ownership of the cell lines developed and their derivatives.
3. Consent for genetic modification of the cell lines.
6. Patent rights for any commercial use of cell lines.
In the general practice of culture techniques using human tissues, the donor and/or relatives are asked to sign a disclaimer statement (in a prescribed pro-forma) before the tissue is taken. By this approach, the legal complications are minimized.
Safety measures:
Handling of human tissues is associated with a heavy risk of exposure for various infections. Therefore, it is absolutely necessary that the human materials are handled in a biohazard cabinet. The tissues should be screened for various infections such as hepatitis, tuberculosis, HIV, before their use. Further, the media and apparatus, after their use must be autoclaved or disinfected, so that the spread of infections is drastically reduced.