ADVERTISEMENTS:
The following points highlight the top two types of membrane models. The types are: 1. Lamellar Models 2. Micellar or Sub-Unit Models.
Membrane Models: Type # 1. Lamellar Models:
(I) Lipid Bilayer Hypothesis:
Overton (1895) suggested that the cell membranes contain lipids. This conclusion was based on the fact that fat solvents dissolved the membrane easily and fat soluble substances passed easily through the cell membranes. Some workers like Hober (1910) and Fricke (1925) found low electrical conductivity in the cell membrane which indicated the presence of lipid layer.
ADVERTISEMENTS:
Gorter and Grendel (1925)’ were the first to suggest a possible structure of the cell membrane. On the basis of studies of cell membranes of erythrocytes they showed that the lipid extracted from the red cell ghost spread in area twice that of a simple molecular film.
So it was thought that the membrane consisted of double layers of lipid molecules, the polar hydrophilic groups of the molecules being situated on the outside and hydrophobic ends standing at right angles to the surface are oriented inwardly (Fig. 2.1).
(II) Protein-lipid-protein Hypothesis (Sandwich Models):
ADVERTISEMENTS:
(i) Danielli and Davson Model:
Harvey and Coley (1931) and Danielli and Harvey (1935) studied surface tension of cell membrane and on the basis of their observation they pointed out the existence of protein molecules adsorbed on the surface of lipid droplets which reduce the surface tension of droplets.
This conclusion led James Danielli and Hugh Davson in 1935 to suggest bimolecular leaflet model of cell membrane. Danielli and Davson model was the first attempt to describe membrane structure in terms of molecules and to relate the structure to biological and chemical properties.
According to bimolecular model of Danielli and Davson, plasma membrane consists of two layers of phospholipid molecules (a bimolecular leaflet) in which phospholipid molecules are arranged in such a way that hydrophilic heads of the phospholipid molecules face outside and hydrophobic non-polar lipid chains are associated in the inner region of leaflet.
The hypothesis also suggested that the polar ends of lipid molecules are associated with monomolecular layer of globular proteins. The plasma membrane would thus consist of a double layer of phospholipid molecules sandwiched between two essentially continuous layers of protein (Fig. 2.2).
This basic model has been modified from time to time. Danielli (1938) suggested the presence of two types of proteins; tangentially arranged in contact with the lipid and globular proteins on the outer surface. Again Davson and Danielli (1943) and Danielli (1954) considered proteins to be in the form of a folded P-chain.
Perhaps, these units form micelles of membranes indicated in recent electron micrographs. Membrane models are usually postulated to contain protein lined polar pores of about 7 Å diameter which probably permit the passage of small ions and water molecules across the membrane.
In still other variations the proteins are thought to be in coiled or globular form on both sides of lipid layers [Fig. 2.3 (A), (B), (C)] or they are thought to be asymmetrical, with a folded P-chain on one side and globular proteins on the other [Fig. 2.3 (D)].
ADVERTISEMENTS:
Models with globular proteins on both the sides or with folded P-proteins on both the surfaces and helical proteins extending into the pores are also suggested.
(ii) Unit Membrane Model:
In 1950 J. David Robertson studied the cell membranes from electron micrographs of sectioned material. The preparations involved usual fixing in solutions of osmium tetraoxide and potassium permanganate (KMnO4), and dehydrating in solvents such as acetone before sectioning.
In late 1950s Robertson summarised a large number of ultra-structural data obtained by him and some other workers and concluded that the plasma membrane and the membranes of all cell organalles were similar in structure. Although the similarity is not resolved by light microscopy, it is clearly seen in electron micrographs.
ADVERTISEMENTS:
This conclusion led Robertson in 1953 to propose unit membrane hypothesis according to which all biological membranes show generalised unit membrane construction. The unit membrane model visualises cell membrane as a trilaminar and indicates structure consisting of two dark osmiophilic layers separated by a light osmiophilic layer.
The physical appearance of this trilaminar model has led to the term unit membrane. The unit membrane concept implies a trilaminar appearance with a bimolecular lipid layer between two protein layers (Fig. 2.4). Each dense osmiophilic band is made up of protein (20 Å) and the polar groups of phospholipids (5 A) and is thus 25 Å thick.
The clear Osmiophilic zone 35 A in thickness is a bimolecular layer of lipids without the polar groups. In other words, the unit membrane is 75 Å thick with a 35 Å thick phospholipid layer between two 20 Å thick protein layers. The plasma membrane surrounding the cell is thicker at the free surfaces of the cell than where it is in contact with other cells.
In unit membrane model the protein layers are assymetrical. On the outer surface it is mucoprotein while on the inner surface it is non-mucoid protein.
ADVERTISEMENTS:
Some recent studies have indicated that the arrangement of lipids and proteins in the cell membranes may be different in different cells. Artificial membranes have been prepared consisting of a bimolecular layers of phospholipids with proteins adsorbed on both the surfaces of lipid layer.
The electron micrographs of such osmium fixed synthetic membranes revealed two dark bands 20-25 Å thick and a clear space of 20-25 Å between them. The appearance of these artificial membranes is much similar to that of plasma membranes.
Robertson’s unit membrane model has been widely accepted as it accounted for a number of properties of cell membranes which are as follows:
1. The densely packed bimolecular lipid layer easily accounted for the presence of 40% lipid by weight in the membrane.
ADVERTISEMENTS:
2. The model accounted for the three layered staining pattern of fixed membranes as observed in electron micrographs.
3. Phospholipids spontaneously form a bimolecular system in vitro when added to an aqueous environment and there is no requirement for work input to maintain the minimum energy conformation of the synthetic membrane.
4. Hydrocarbons are poor electrical conductors, so the continuous hydrocarbon phase of the natural membrane would explain the known high electrical resistance of membranes.
5. High permeability of natural membranes to non-polar molecules could be explained by their solubility in the nonpolar lipid phase and at the same time accounted for relative impermeability to small ions which do not dissolve readily in this medium.
Some objections to the unit membrane model were advanced during 1960s which are as follows:
(i) Unit membrane concept required the uniformity in the feature of membrane but mitichondrial, endoplasmic reticular and plastid membranes have been shown by F.S. Sjostrand (1963) to be different from the plasma membrane.
ADVERTISEMENTS:
(ii) Mitochondrial and plastid membranes display particulate units in or on them but the plasma membrane does not present such appearance.
(iii) The unit membrane appears as a single trilaminar structure in plasma membrane, endoplasmic reticulum, golgi complex and lysosomes but in mitochondria, plastids and nuclei, the boundaries have an outer and an inner membrane, each with unit membrane structural plan.
(iv) There can be some variations in the thickness of the unit membranes as a result of varying thickness of osmiophilic and Osmiophobic layers.
It is believed that the outer and inner surfaces of membrane are different. The inner surface of membrane is thought to be made up of unconjugated protein and the outer surface layer of conjugated protein and polysaccharides, mucoproteins. Attached to the glycoproteins are oligosaccharides-side chains with negatively charged sialic acid terminals.
Thus the membrane is asymmetrical in organisation and shows structural polarity (Figs. 2.3 A-F and 2.4). Due to this feature unit membrane model is also called greater membrane model.
(iii) Kavanau’s Lipid Pillar Model:
This is a modification of Danielli and Davson trilaminar sandwich model in which lipid layer is visualised in two forms. In one case, it is in the form of pillar while in others it is in the form of flattened discs. The spaces between the pillars act as pores for the passage of ions.
The interior of each pillar is formed of non-polar faces of phospholipids while the surface is formed of the polar heads. Protein layers are present on both sides of membrane. The main drawback of this model is that it cannot explain active transport or the differential permeability of Na+ or K+ ions.
(IV) Models in which proteins are considered to penetrate lipid layers:
According to some workers, proteins are considered to penetrate the lipid layer. This model can explain the low surface tension of biological membrane as properly as the trilaminar model.
1. Benson’s Model:
Benson (1966) proposed a cell membrane model on the basis of a study of chloroplast membrane. According to him, the lipids and proteins form a hydrophobic association. The charged polar heads of phospholipid molecules lie on the surface of membrane and are capable of binding ions. The non-polar hydrophobic lipid tails are linked with complementary hydrophobic regions within the interior of proteins.
2. Lanard and Singers Model:
This model was proposed in 1966. According to this model, one- fourth of the proteins are in helical conformation and the rest form random coils.
3. Mosaic membrane concept:
According to Baum 1967, “The backbone of membrane is possibly formed by toast shaped cuboidal units of about 80 Å diameter which are covered along the edges as well as the sides by phospholipids”. The heads of phospholipids face on the outside while the tails form complexes with the hydrophobic surfaces of the proteins.
4. Fluid Mosaic Model:
The fluid mosaic model of cell membrane was proposed in 1972 by S.J. Singer and G.L. Nicolson. According to this model, the cell membranes have been visualised as mosaics of lipids and proteins. The lipids are thought to be arranged primarily in a bilayer in which peripheral and integral proteins are embedded to varying degrees (Fig. 2.5).
Immunological experiments have indicated the attachment of antibodies to surface-exposed integral proteins such as glycophorine.
This suggests that membrane proteins are not fixed within the lipid layer but are free to move laterally like icebergs floating in a sea of lipids. This picture has inspired Singer and Nicolson to coin fluid mosaic model.
If this analogy of icebergs floating in a sea of lipids is valid then it should be possible to freeze proteins by solidifying the lipid sea in which they float, his is indeed, possible when the phase transition occurs from liquid to solid. At this point the mobility of proteins in the membrane will be checked.
Singer and Nicolson considered the lipoprotein association to be hydrophobic and fluidity of the membrane results due to hydrophobic interaction. It should be noted that phospholipids and many intrinsic proteins are amphipatic molecules, i.e., both hydrophilic and hydrophobic groups occur within the same molecule.
The globular proteins of the membrane are of two different types: extrinsic (peripheral protein) and intrinsic (integral proteins).
Because of rapid movement of lipid and protein molecules, the fluid mosaic model is different from the static picture of the membrane in Danielli and Davson model. The proteins of the membrane are concerned with the enzymatic activities, transport of molecules and with receptor function. The lipid bilayer acts as the permeability barrier.
The fluidity of lipid is supported by many indirect studies based on x-ray diffraction, differential thermal analysis and electron spin resonance (ESR) techniques.
These techniques indicate that lipid bilayer has many dynamic properties which are as follows:
1. The rapid motion involving flaxing within each lipid molecule is possible.
2. A rapid lateral diffusion of lipid is possible.
3. Slow motion of lipid molecule from one side of the bilayer to the other is also possible.
4. The lipid molecules might rotate about their axis.
The fluid mosaic model of cell membrane is now widely accepted as it is presumed to apply to membranes of all types regardless of their varying characteristics and differences in lipids protein ratio. In fact this model can account for the molecular organisation and ultrastructure of membranes in-terms of their chemical composition.
Membrane Models: Type # 2. Micellar Model:
Hilleir and Hofman (1953) have suggested a molecular structure of biological membrane in which the membrane has been considered to be a non-laminar pattern and consisting of globular sub-units known as micelles which have a lipid core and a hydrophobic shell of polar groups. Lipid micelles are possible building blocks of membranes [Figs. 2.3 (E), (F)].
In this model protein component may form a monolayer on either side of the plane of lipid micelles. Individual sub-unit might be replaced by individual enzyme molecule or by array of enzymes with a precise three dimensional organization.
The spaces between the micelles are thought to form water filled pores of about 4 Å diameter lined partly by polar groups of a micelles and partly by polar groups of associated proteins molecule.
The high resolution electron micrographs lend some support to both lamellar and micellar hypotheses for membrane structure yet they do not provide any convincing evidence for either which might resolve this controversy.
Some workers have postulated that intracellular membranes have micellar structure while the plasma membrane has lamellar configuration and there occurs a mutual transformation between the two. Fernandez Moren (1962) and Sjostrand considered the membrane to be composed of globular units or elementary particles.
The globules, 40-70 Å in diameter, form repeating units which are closely packed together but this does not hold good for all membranes. Some workers considered corpuscular nature to be a fact.
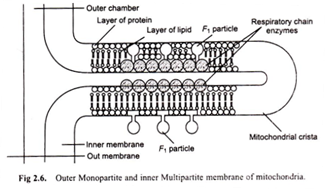
According to Green (1960), “The cell membranes consist of fused repeating units (Fig. 2.6)”. Two types of membranes are distinguished monopartite and multipartite.
In monopartite membrane, the repeating units do not have any projections. Examples of such membranes are the outer membranes of mitochondria and plasma membrane.
In multipartite membranes, the repeating unit is divisible into a base piece, a stalk and head piece. The monopartite repeating units correspond to the base piece of multipartite units. Multipartite membranes are composed of repeating units of two types; macrotripartite and microtripartite units, the former being larger than the latter ones.
The macrotripartite units are found in membranes in which electron transfer is coupled with ATP synthesis, e.g., the inner membrane of mitochondria. Membranes with micropartite units have the capacity to carry out ATP energised active transport, e.g., the plasma membrane which transports K+ and Na+ and the microvilli which transport sugar and amino acids.
In macrotripartite membrane ATPase function is localized in the head pieces while in the micropartite membranes, it is localized in the base pieces.
Protein Crystal Model:
David Green and co-workers (1970) have proposed a protein crystal model for cell membrane in which proteins polymerise to form two layers of loosely packed globular protein units of 30-40 Å diameter. The proteins are visualised as having extensive non-polar as well as polar regions on their surfaces.
The non-polar region may exhibit hydrophobic bonding with non-polar groups of phospholipid molecules that fill the cavities between the globular protein units while the polar heads of phospholipid molecules face the membrane surface (Fig. 2.7).
This model accounts for some of the observed chemical and biological characteristics of membranes. Green has recently modified the protein crystal model toward a more dynamic model in which proteins and lipids are considered to move in the plane of membrane.
Green points out that a consequence of this modification is that the lipid layer forms essentially a continuum and protein- protein interactions are only temporary.